Human cytomegalovirus IE1-72 activates ataxia telangiectasia mutated kinase and a p53/p21-mediated growth arrest response
- PMID: 16103197
- PMCID: PMC1193638
- DOI: 10.1128/JVI.79.17.11467-11475.2005
Human cytomegalovirus IE1-72 activates ataxia telangiectasia mutated kinase and a p53/p21-mediated growth arrest response
Abstract
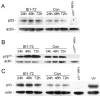
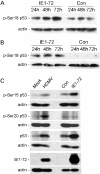
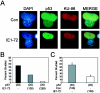
Human cytomegalovirus (HCMV) encodes several proteins that can modulate components of the cell cycle machinery. The UL123 gene product, IE1-72, binds the Rb-related, p107 protein and relieves its repression of E2F-responsive promoters; however, it is unable to induce quiescent cells to enter S phase in wild-type (p53(+/+)) cells. IE1-72 also induces p53 accumulation through an unknown mechanism. We present here evidence suggesting that IE1-72 may activate the p53 pathway by increasing the levels of p19(Arf) and by inducing the phosphorylation of p53 at Ser15. Phosphorylation of this residue by IE1-72 expression alone or HCMV infection is found to be dependent on the ataxia-telangiectasia mutated kinase. IE2-86 expression leads to p53 phosphorylation and may contribute to this phenotype in HCMV-infected cells. We also found that IE1-72 promotes p53 nuclear accumulation by abrogating p53 nuclear shuttling. These events result in the stimulation of p53 activity, leading to a p53- and p21-dependent inhibition of cell cycle progression from G(1) to S phase in cells transiently expressing IE1-72. Thus, like many of the small DNA tumor viruses, the first protein expressed upon HCMV infection activates a p53 response by the host cell.
Figures

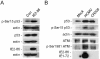


Similar articles
-
Human cytomegalovirus disrupts both ataxia telangiectasia mutated protein (ATM)- and ATM-Rad3-related kinase-mediated DNA damage responses during lytic infection.J Virol. 2007 Feb;81(4):1934-50. doi: 10.1128/JVI.01670-06. Epub 2006 Dec 6. J Virol. 2007. PMID: 17151099 Free PMC article.
-
Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection.Virology. 2000 Aug 15;274(1):39-55. doi: 10.1006/viro.2000.0448. Virology. 2000. PMID: 10936087
-
Human cytomegalovirus IE1-72 protein interacts with p53 and inhibits p53-dependent transactivation by a mechanism different from that of IE2-86 protein.J Virol. 2009 Dec;83(23):12388-98. doi: 10.1128/JVI.00304-09. Epub 2009 Sep 23. J Virol. 2009. PMID: 19776115 Free PMC article.
-
Nitric oxide and p53 in cancer-prone chronic inflammation and oxyradical overload disease.Environ Mol Mutagen. 2004;44(1):3-9. doi: 10.1002/em.20024. Environ Mol Mutagen. 2004. PMID: 15199542 Review.
-
Checking in on hypoxia/reoxygenation.Cell Cycle. 2006 Jun;5(12):1304-7. doi: 10.4161/cc.5.12.2811. Epub 2006 Jun 15. Cell Cycle. 2006. PMID: 16760660 Review.
Cited by
-
Early Nuclear Events after Herpesviral Infection.J Clin Med. 2019 Sep 7;8(9):1408. doi: 10.3390/jcm8091408. J Clin Med. 2019. PMID: 31500286 Free PMC article. Review.
-
Human Cytomegalovirus miR-UL148D Facilitates Latent Viral Infection by Targeting Host Cell Immediate Early Response Gene 5.PLoS Pathog. 2016 Nov 8;12(11):e1006007. doi: 10.1371/journal.ppat.1006007. eCollection 2016 Nov. PLoS Pathog. 2016. PMID: 27824944 Free PMC article.
-
The DNA damage response induced by infection with human cytomegalovirus and other viruses.Viruses. 2014 May 23;6(5):2155-85. doi: 10.3390/v6052155. Viruses. 2014. PMID: 24859341 Free PMC article. Review.
-
The Role of Caspase-12 in Retinal Bystander Cell Death and Innate Immune Responses against MCMV Retinitis.Int J Mol Sci. 2021 Jul 29;22(15):8135. doi: 10.3390/ijms22158135. Int J Mol Sci. 2021. PMID: 34360899 Free PMC article.
-
Human cytomegalovirus riding the cell cycle.Med Microbiol Immunol. 2015 Jun;204(3):409-19. doi: 10.1007/s00430-015-0396-z. Epub 2015 Mar 17. Med Microbiol Immunol. 2015. PMID: 25776080 Review.
References
-
- Abraham, R. T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177-2196. - PubMed
-
- Ahn, J. H., and G. S. Hayward. 2000. Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection. Virology 274:39-55. - PubMed
-
- Ahn, J. H., W. J. Jang, and G. S. Hayward. 1999. The human cytomegalovirus IE2 and UL112-113 proteins accumulate in viral DNA replication compartments that initiate from the periphery of promyelocytic leukemia protein-associated nuclear bodies (PODs or ND10). J. Virol. 73:10458-10471. - PMC - PubMed
-
- Bakkenist, C. J., and M. B. Kastan. 2003. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421:499-506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

