Key Golgi factors for structural and functional maturation of bunyamwera virus
- PMID: 16103138
- PMCID: PMC1193595
- DOI: 10.1128/JVI.79.17.10852-10863.2005
Key Golgi factors for structural and functional maturation of bunyamwera virus
Abstract
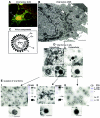
Several complex enveloped viruses assemble in the membranes of the secretory pathway, such as the Golgi apparatus. Among them, bunyaviruses form immature viral particles that change their structure in a trans-Golgi-dependent manner. To identify key Golgi factors for viral structural maturation, we have purified and characterized the three viral forms assembled in infected cells, two intracellular intermediates and the extracellular mature virion. The first viral form is a pleomorphic structure with fully endo-beta-N-acetylglucosaminidase H (Endo-H)-sensitive, nonsialylated glycoproteins. The second viral intermediate is a structure with hexagonal and pentagonal contours and partially Endo-H-resistant glycoproteins. Sialic acid is incorporated into the small glycoprotein of this second viral form. Growing the virus in glycosylation-deficient cells confirmed that acquisition of Endo-H resistance but not sialylation is critical for the trans-Golgi-dependent structural maturation and release of mature viruses. Conformational changes in viral glycoproteins triggered by changes in sugar composition would then induce the assembly of a compact viral particle of angular contours. These structures would be competent for the second maturation step, taking place during exit from cells, that originates fully infectious virions.
Figures

Similar articles
-
Polymorphism and structural maturation of bunyamwera virus in Golgi and post-Golgi compartments.J Virol. 2003 Jan;77(2):1368-81. doi: 10.1128/jvi.77.2.1368-1381.2003. J Virol. 2003. PMID: 12502853 Free PMC article.
-
Inhibition of host ER glucosidase activity prevents Golgi processing of virion-associated bovine viral diarrhea virus E2 glycoproteins and reduces infectivity of secreted virions.Virology. 2002 Mar 30;295(1):10-9. doi: 10.1006/viro.2002.1370. Virology. 2002. PMID: 12033761
-
Mapping the Golgi targeting and retention signal of Bunyamwera virus glycoproteins.J Virol. 2004 Oct;78(19):10793-802. doi: 10.1128/JVI.78.19.10793-10802.2004. J Virol. 2004. PMID: 15367646 Free PMC article.
-
Targeting of viral glycoproteins to the Golgi complex.Trends Microbiol. 1993 Jul;1(4):124-30. doi: 10.1016/0966-842x(93)90126-c. Trends Microbiol. 1993. PMID: 8143127 Free PMC article. Review.
-
Virus maturation.Subcell Biochem. 2013;68:395-415. doi: 10.1007/978-94-007-6552-8_13. Subcell Biochem. 2013. PMID: 23737059 Review.
Cited by
-
Bunyamwera virus possesses a distinct nucleocapsid protein to facilitate genome encapsidation.Proc Natl Acad Sci U S A. 2013 May 28;110(22):9048-53. doi: 10.1073/pnas.1222552110. Epub 2013 Apr 8. Proc Natl Acad Sci U S A. 2013. PMID: 23569257 Free PMC article.
-
Oropouche Virus Glycoprotein Topology and Cellular Requirements for Glycoprotein Secretion.J Virol. 2023 Jan 31;97(1):e0133122. doi: 10.1128/jvi.01331-22. Epub 2022 Dec 8. J Virol. 2023. PMID: 36475765 Free PMC article.
-
Nucleocapsid protein structures from orthobunyaviruses reveal insight into ribonucleoprotein architecture and RNA polymerization.Nucleic Acids Res. 2013 Jun;41(11):5912-26. doi: 10.1093/nar/gkt268. Epub 2013 Apr 17. Nucleic Acids Res. 2013. PMID: 23595147 Free PMC article.
-
Novel replication complex architecture in rubella replicon-transfected cells.Cell Microbiol. 2007 Apr;9(4):875-90. doi: 10.1111/j.1462-5822.2006.00837.x. Epub 2006 Nov 3. Cell Microbiol. 2007. PMID: 17087733 Free PMC article.
-
Orthobunyavirus ultrastructure and the curious tripodal glycoprotein spike.PLoS Pathog. 2013;9(5):e1003374. doi: 10.1371/journal.ppat.1003374. Epub 2013 May 16. PLoS Pathog. 2013. PMID: 23696739 Free PMC article.
References
-
- Altan-Bonnet, N., R. Sougrat, and J. Lippincott-Schwartz. 2004. Molecular basis for Golgi maintenance and biogenesis. Curr. Opin. Cell Biol. 16:364-372. - PubMed
-
- Baumeister, W. 2002. Electron tomography: towards visualizing the molecular organization of the cytoplasm. Curr. Opin. Struct. Biol. 12:679-684. - PubMed
-
- Bijlmakers, M. J., and M. Marsh. 2003. The on-off story of protein palmitoylation. Trends Cell Biol. 13:32-42. - PubMed
-
- Bonay, P., S. Munro, M. Fresno, and B. Alarcon. 1996. Intra-Golgi transport inhibition by megalomycin. J. Biol. Chem. 271:3719-3726. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

