Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients
- PMID: 16099790
- PMCID: PMC1774653
- DOI: 10.1136/gut.2004.061440
Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients
Abstract
Background and aims: Glucagon-like peptide 2 (GLP-2) may improve intestinal absorption in short bowel syndrome (SBS) patients with an end jejunostomy. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant GLP-2 analogue, prolongs the intestinotrophic properties of GLP-2 in animal models. The safety and effect of teduglutide were investigated in SBS patients with and without a colon in continuity.
Methods: Teduglutide was given subcutaneously for 21 days once or twice daily to 16 SBS patients in the per protocol investigational group, 10 with end jejunostomy (doses of 0.03 (n = 2), 0.10 (n = 5), or 0.15 (n = 3) mg/kg/day), one with <50% colon in continuity (dose 0.03 mg/kg/day), and five with > or = 50% colon in continuity (dose 0.10 mg/kg/day). Nutrient balance studies, D-xylose tests, and intestinal mucosa biopsies were performed at baseline, on the last three days of treatment, and after three weeks of follow up. Pre-study fasting native GLP-2 levels were determined for the five patients with > or = 50% colon in continuity.
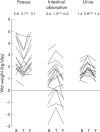
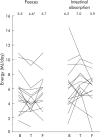
Results: Pooled across groups and compared with baseline, teduglutide increased absolute (+743 (477) g/day; p<0.001) and relative (+22 (16)%; p<0.001) wet weight absorption, urine weight (+555 (485) g/day; p<0.001), and urine sodium excretion (+53 (40) mmol/day; p<0.001). Teduglutide decreased faecal wet weight (-711 (734) g/day; p = 0.001) and faecal energy excretion (-808 (1453) kJ/day (-193 (347) kcal/day); p = 0.040). In SBS patients with end jejunostomy, teduglutide significantly increased villus height (+38 (45)%; p = 0.030), crypt depth (+22 (18)%; p = 0.010), and mitotic index (+115 (108)%; p = 0.010). Crypt depth and mitotic index did not change in colonic biopsies from SBS patients with colon in continuity. The most common side effects were enlargement of the stoma nipple and mild lower leg oedema. The improvements in intestinal absorption and decreases in faecal excretion noted after treatment had reversed after the drug free follow up period. A controlled study with a more robust design is ongoing in order to determine the optimal dosage of teduglutide for SBS patients to achieve the maximal effect and utility of this drug in clinical practice.
Conclusion: Teduglutide, at three dose levels for 21 days, was safe and well tolerated, intestinotrophic, and significantly increased intestinal wet weight absorption in SBS patients with an end jejunostomy or a colon in continuity.
Figures
Similar articles
-
Acute effects of the glucagon-like peptide 2 analogue, teduglutide, on intestinal adaptation in short bowel syndrome.J Pediatr Gastroenterol Nutr. 2014 Jun;58(6):694-702. doi: 10.1097/MPG.0000000000000295. J Pediatr Gastroenterol Nutr. 2014. PMID: 24399211
-
Quality of life in patients with short bowel syndrome treated with the new glucagon-like peptide-2 analogue teduglutide--analyses from a randomised, placebo-controlled study.Clin Nutr. 2013 Oct;32(5):713-21. doi: 10.1016/j.clnu.2013.03.016. Epub 2013 Mar 28. Clin Nutr. 2013. PMID: 23587733 Clinical Trial.
-
Factors Associated With Response to Teduglutide in Patients With Short-Bowel Syndrome and Intestinal Failure.Gastroenterology. 2018 Mar;154(4):874-885. doi: 10.1053/j.gastro.2017.11.023. Epub 2017 Nov 22. Gastroenterology. 2018. PMID: 29174926 Clinical Trial.
-
New approaches to the treatments of short bowel syndrome-associated intestinal failure.Curr Opin Gastroenterol. 2014 Mar;30(2):182-8. doi: 10.1097/MOG.0000000000000046. Curr Opin Gastroenterol. 2014. PMID: 24406477 Review.
-
Pharmacologic options for intestinal rehabilitation in patients with short bowel syndrome.JPEN J Parenter Enteral Nutr. 2014 May;38(1 Suppl):45S-52S. doi: 10.1177/0148607114526241. Epub 2014 Mar 10. JPEN J Parenter Enteral Nutr. 2014. PMID: 24615689 Review.
Cited by
-
Enteral nutrients potentiate glucagon-like peptide-2 action and reduce dependence on parenteral nutrition in a rat model of human intestinal failure.Am J Physiol Gastrointest Liver Physiol. 2012 Sep 1;303(5):G610-22. doi: 10.1152/ajpgi.00184.2012. Epub 2012 Jun 28. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22744334 Free PMC article.
-
An updated overview of glucagon-like peptide-2 analog trophic therapy for short bowel syndrome in adults.J Int Med Res. 2022 Mar;50(3):3000605221086145. doi: 10.1177/03000605221086145. J Int Med Res. 2022. PMID: 35343263 Free PMC article. Review.
-
Regulation of epithelial differentiation in rat intestine by intraluminal delivery of an adenoviral vector or silencing RNA coding for Schlafen 3.PLoS One. 2013 Nov 11;8(11):e79745. doi: 10.1371/journal.pone.0079745. eCollection 2013. PLoS One. 2013. PMID: 24244554 Free PMC article.
-
Glucagon-like peptide-2 does not modify the growth or survival of murine or human intestinal tumor cells.Cancer Res. 2008 Oct 1;68(19):7897-904. doi: 10.1158/0008-5472.CAN-08-0029. Cancer Res. 2008. PMID: 18829546 Free PMC article.
-
The pharmacologic treatment of short bowel syndrome: new tricks and novel agents.Curr Gastroenterol Rep. 2014;16(7):392. doi: 10.1007/s11894-014-0392-2. Curr Gastroenterol Rep. 2014. PMID: 25052938 Review.
References
-
- Howard L, Ament M, Fleming CR, et al. Current use and clinical outcome of home parenteral and enteral nutrition therapies in the United States. Gastroenterology 1995;109:355–65. - PubMed
-
- Buchman AL, Scolapio J, Fryer J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology 2003;124:1111–34. - PubMed
-
- Bakker H, Bozzetti F, Staun M, et al. Home parenteral nutrition in adults: a european multicentre survey in 1997. ESPEN-Home Artificial Nutrition Working Group. Clin Nutr 1999;18:135–40. - PubMed
-
- Howard L, Ashley C. Management of complications in patients receiving home parenteral nutrition. Gastroenterology 2003;124:1651–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
