A microfluidic culture platform for CNS axonal injury, regeneration and transport
- PMID: 16094385
- PMCID: PMC1558906
- DOI: 10.1038/nmeth777
A microfluidic culture platform for CNS axonal injury, regeneration and transport
Abstract
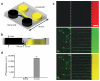
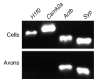
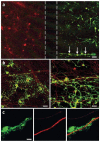
Investigation of axonal biology in the central nervous system (CNS) is hindered by a lack of an appropriate in vitro method to probe axons independently from cell bodies. Here we describe a microfluidic culture platform that polarizes the growth of CNS axons into a fluidically isolated environment without the use of targeting neurotrophins. In addition to its compatibility with live cell imaging, the platform can be used to (i) isolate CNS axons without somata or dendrites, facilitating biochemical analyses of pure axonal fractions and (ii) localize physical and chemical treatments to axons or somata. We report the first evidence that presynaptic (Syp) but not postsynaptic (Camk2a) mRNA is localized to developing rat cortical and hippocampal axons. The platform also serves as a straightforward, reproducible method to model CNS axonal injury and regeneration. The results presented here demonstrate several experimental paradigms using the microfluidic platform, which can greatly facilitate future studies in axonal biology.
Figures


Similar articles
-
Axon length quantification microfluidic culture platform for growth and regeneration study.Methods Mol Biol. 2014;1162:85-95. doi: 10.1007/978-1-4939-0777-9_7. Methods Mol Biol. 2014. PMID: 24838960 Free PMC article.
-
Mouse hippocampal explant culture system to study isolated axons.J Neurosci Methods. 2014 Jul 30;232:157-64. doi: 10.1016/j.jneumeth.2014.05.018. Epub 2014 May 24. J Neurosci Methods. 2014. PMID: 24861423
-
Live Imaging Analysis of Axonal Regeneration in Human iPSC-Derived Motor Neurons Using a Microfluidic System.Methods Mol Biol. 2024;2831:333-350. doi: 10.1007/978-1-0716-3969-6_23. Methods Mol Biol. 2024. PMID: 39134861
-
Microfluidic and compartmentalized platforms for neurobiological research.Crit Rev Biomed Eng. 2011;39(3):185-200. doi: 10.1615/critrevbiomedeng.v39.i3.20. Crit Rev Biomed Eng. 2011. PMID: 21967302 Review.
-
Integrated microfluidics platforms for investigating injury and regeneration of CNS axons.Ann Biomed Eng. 2012 Jun;40(6):1268-76. doi: 10.1007/s10439-012-0515-6. Ann Biomed Eng. 2012. PMID: 22302320 Review.
Cited by
-
Visualizing K48 Ubiquitination during Presynaptic Formation By Ubiquitination-Induced Fluorescence Complementation (UiFC).Front Mol Neurosci. 2016 Jun 10;9:43. doi: 10.3389/fnmol.2016.00043. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27375430 Free PMC article.
-
Neuronal models of TDP-43 proteinopathy display reduced axonal translation, increased oxidative stress, and defective exocytosis.Front Cell Neurosci. 2023 Nov 13;17:1253543. doi: 10.3389/fncel.2023.1253543. eCollection 2023. Front Cell Neurosci. 2023. PMID: 38026702 Free PMC article.
-
Design and implementation of in vivo imaging of neural injury responses in the adult Drosophila wing.Nat Protoc. 2013 Apr;8(4):810-9. doi: 10.1038/nprot.2013.042. Nat Protoc. 2013. PMID: 23589940 Free PMC article.
-
Axon degeneration: context defines distinct pathways.Curr Opin Neurobiol. 2016 Aug;39:108-15. doi: 10.1016/j.conb.2016.05.002. Epub 2016 May 16. Curr Opin Neurobiol. 2016. PMID: 27197022 Free PMC article. Review.
-
Neuronal activity enhances tau propagation and tau pathology in vivo.Nat Neurosci. 2016 Aug;19(8):1085-92. doi: 10.1038/nn.4328. Epub 2016 Jun 20. Nat Neurosci. 2016. PMID: 27322420 Free PMC article.
References
-
- Terry R, et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30:572–580. - PubMed
-
- McKerracher L. Spinal cord repair: strategies to promote axon regeneration. Neurobiol. Dis. 2001;8:11–18. - PubMed
-
- Medana IM, Esiri MM. Axonal damage: a key predictor of outcome in human CNS diseases. Brain. 2003;126:515–530. - PubMed
-
- Salehi A, Delcroix JD, Mobley WC. Traffic at the intersection of neurotrophic factor signaling and neurodegeneration. Trends Neurosci. 2003;26:73–80. - PubMed
-
- MacInnis BL, Campenot RB. Retrograde support of neuronal survival without retrograde transport of nerve growth factor. Science. 2002;295:1536–1539. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

