Synaptotagmin isoforms couple distinct ranges of Ca2+, Ba2+, and Sr2+ concentration to SNARE-mediated membrane fusion
- PMID: 16093350
- PMCID: PMC1237081
- DOI: 10.1091/mbc.e05-04-0277
Synaptotagmin isoforms couple distinct ranges of Ca2+, Ba2+, and Sr2+ concentration to SNARE-mediated membrane fusion
Abstract
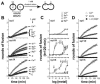
Ca2+-triggered exocytosis of synaptic vesicles is controlled by the Ca2+-binding protein synaptotagmin (syt) I. Fifteen additional isoforms of syt have been identified. Here, we compared the abilities of three syt isoforms (I, VII, and IX) to regulate soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-mediated membrane fusion in vitro in response to divalent cations. We found that different isoforms of syt couple distinct ranges of Ca2+, Ba2+, and Sr2+ to membrane fusion; syt VII was approximately 400-fold more sensitive to Ca2+ than was syt I. Omission of phosphatidylserine (PS) from both populations of liposomes completely abrogated the ability of all three isoforms of syt to stimulate fusion. Mutations that selectively inhibit syt.target-SNARE (t-SNARE) interactions reduced syt stimulation of fusion. Using Sr2+ and Ba2+, we found that binding of syt to PS and t-SNAREs can be dissociated from activation of fusion, uncovering posteffector-binding functions for syt. Our data demonstrate that different syt isoforms are specialized to sense different ranges of divalent cations and that PS is an essential effector of Ca2+.syt action.
Figures



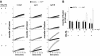
Similar articles
-
Regulation of exocytosis and fusion pores by synaptotagmin-effector interactions.Mol Biol Cell. 2010 Aug 15;21(16):2821-31. doi: 10.1091/mbc.E10-04-0285. Epub 2010 Jun 23. Mol Biol Cell. 2010. PMID: 20573977 Free PMC article.
-
Ca(2+)-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion.Nat Struct Mol Biol. 2006 Apr;13(4):323-30. doi: 10.1038/nsmb1076. Epub 2006 Mar 26. Nat Struct Mol Biol. 2006. PMID: 16565726
-
Analysis of the synaptotagmin family during reconstituted membrane fusion. Uncovering a class of inhibitory isoforms.J Biol Chem. 2008 Aug 1;283(31):21799-807. doi: 10.1074/jbc.M709628200. Epub 2008 May 28. J Biol Chem. 2008. PMID: 18508778 Free PMC article.
-
Fast, Ca2+-dependent exocytosis at nerve terminals: shortcomings of SNARE-based models.Prog Neurobiol. 2014 Oct;121:55-90. doi: 10.1016/j.pneurobio.2014.07.001. Epub 2014 Jul 17. Prog Neurobiol. 2014. PMID: 25042638 Review.
-
There's more to life than neurotransmission: the regulation of exocytosis by synaptotagmin VII.Trends Cell Biol. 2005 Nov;15(11):626-31. doi: 10.1016/j.tcb.2005.09.001. Epub 2005 Sep 15. Trends Cell Biol. 2005. PMID: 16168654 Review.
Cited by
-
Loss of synaptotagmin IV results in a reduction in synaptic vesicles and a distortion of the Golgi structure in cultured hippocampal neurons.Neuroscience. 2010 Apr 28;167(1):135-42. doi: 10.1016/j.neuroscience.2010.01.056. Epub 2010 Feb 4. Neuroscience. 2010. PMID: 20138128 Free PMC article.
-
Synaptotagmin 9 Modulates Spontaneous Neurotransmitter Release in Striatal Neurons by Regulating Substance P Secretion.J Neurosci. 2023 Mar 1;43(9):1475-1491. doi: 10.1523/JNEUROSCI.1857-22.2023. Epub 2023 Feb 2. J Neurosci. 2023. PMID: 36732068 Free PMC article.
-
The Janus-faced nature of the C(2)B domain is fundamental for synaptotagmin-1 function.Nat Struct Mol Biol. 2008 Nov;15(11):1160-8. doi: 10.1038/nsmb.1508. Epub 2008 Oct 26. Nat Struct Mol Biol. 2008. PMID: 18953334 Free PMC article.
-
Synaptotagmin-7-mediated activation of spontaneous NMDAR currents is disrupted in bipolar disorder susceptibility variants.PLoS Biol. 2021 Jul 6;19(7):e3001323. doi: 10.1371/journal.pbio.3001323. eCollection 2021 Jul. PLoS Biol. 2021. PMID: 34228711 Free PMC article.
-
Simulations of active zone structure and function at mammalian NMJs predict that loss of calcium channels alone is not sufficient to replicate LEMS effects.J Neurophysiol. 2023 May 1;129(5):1259-1277. doi: 10.1152/jn.00404.2022. Epub 2023 Apr 19. J Neurophysiol. 2023. PMID: 37073966 Free PMC article.
References
-
- Aravanis, A. M., Pyle, J. L., and Tsien, R. W. (2003). Single synaptic vesicles fusing transiently and successively without loss of identity. Nature 423, 623-627. - PubMed
-
- Augustine, G. J. (2001). How does calcium trigger neurotransmitter release? Curr. Opin. Neurobiol. 11, 320-326. - PubMed
-
- Bai, J., Tucker, W. C., and Chapman, E. R. (2004a). PIP2 increases the speed-of-response of synaptotagmin and steers its membrane penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 11, 36-44. - PubMed
-
- Bai, J., Wang, C. T., Richards, D. A., Jackson, M. B., and Chapman, E. R. (2004b). Fusion pore dynamics are regulated by synaptotagmin*t-SNARE interactions. Neuron 41, 929-942. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

