Anti-lipid phosphate phosphohydrolase-3 (LPP3) antibody inhibits bFGF- and VEGF-induced capillary morphogenesis of endothelial cells
- PMID: 16076388
- PMCID: PMC1201157
- DOI: 10.1186/1478-811X-3-9
Anti-lipid phosphate phosphohydrolase-3 (LPP3) antibody inhibits bFGF- and VEGF-induced capillary morphogenesis of endothelial cells
Abstract
Background: Angiogenesis, or the remodeling of existing vasculature serves as a lifeline to nourish developing embryos and starved tissues, and to accelerate wound healing, diabetic retinopathy, and tumor progression. Recent studies indicate that angiogenesis requires growth factor activity as well as cell adhesion events mediated by alpha5beta1 and alphavbeta3 integrins. We previously demonstrated that human lipid phosphate phosphohydrolase-3 (LPP3) acts as a cell-associated ligand for alpha5beta1 and alphavbeta3 integrins. Here, we test the hypothesis that an anti-LPP3 antibody can inhibit basic fibroblast growth factor (bFGF)-and vascular endothelial growth factor (VEGF)-induced capillary morphogenesis of endothelial cells (ECs).
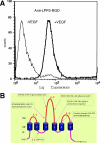
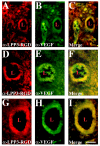
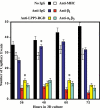
Results: We report that bFGF and VEGF up-regulate LPP3 protein expression in ECs. Immunoprecipitation analyses show that LPP3 is a cell surface protein and undergoes N-glycosylation. Fluorescent activated cell sorting (FACS) data suggest that anti-LPP3-RGD detects native neoepitope on the surface of activated ECs. Moreover, we demonstrate LPP3 protein expression in tumor endothelium alongside VEGF. The embedding of ECs into three-dimensional type I collagen in the presence of bFGF and VEGF induce capillary formation. Importantly, we show that the addition of an anti-LPP3 antibody specifically and significantly blocks bFGF- and VEGF-induced capillary morphogenesis of ECs.
Conclusion: These data suggest that activated ECs as well as tumor endothelium express LPP3 protein. In an in vitro assay, the anti-LPP3-RGD specifically blocks bFGF and VEGF induced capillary morphogenesis of ECs. Our results, therefore, suggest a role for LPP3 in angiogenesis.
Figures


Similar articles
-
Murine lipid phosphate phosphohydrolase-3 acts as a cell-associated integrin ligand.Biochem Biophys Res Commun. 2005 Sep 30;335(3):906-19. doi: 10.1016/j.bbrc.2005.07.157. Biochem Biophys Res Commun. 2005. PMID: 16099422
-
Lipid phosphate phosphatase 3 stabilization of beta-catenin induces endothelial cell migration and formation of branching point structures.Mol Cell Biol. 2010 Apr;30(7):1593-606. doi: 10.1128/MCB.00038-09. Epub 2010 Feb 1. Mol Cell Biol. 2010. PMID: 20123964 Free PMC article.
-
Synergistic effects of vascular endothelial growth factor and basic fibroblast growth factor on the proliferation and cord formation of bovine capillary endothelial cells within collagen gels.Lab Invest. 1993 Nov;69(5):508-17. Lab Invest. 1993. PMID: 8246443
-
Endothelial and glial cell interaction in diabetic retinopathy via the function of vascular endothelial growth factor (VEGF).Pol J Pharmacol. 1996 May-Jun;48(3):307-16. Pol J Pharmacol. 1996. PMID: 9112668 Review.
-
Role of growth factors in coronary morphogenesis.Tex Heart Inst J. 2002;29(4):250-4. Tex Heart Inst J. 2002. PMID: 12484608 Free PMC article. Review.
Cited by
-
PERK Integrates Oncogenic Signaling and Cell Survival During Cancer Development.J Cell Physiol. 2016 Oct;231(10):2088-96. doi: 10.1002/jcp.25336. Epub 2016 Mar 6. J Cell Physiol. 2016. PMID: 26864318 Free PMC article. Review.
-
Requirement of alpha(4)beta(1) and alpha(5)beta(1) integrin expression in bone-marrow-derived progenitor cells in preventing endotoxin-induced lung vascular injury and edema in mice.Stem Cells. 2009 Dec;27(12):3112-20. doi: 10.1002/stem.241. Stem Cells. 2009. PMID: 19839056 Free PMC article.
-
Mice with targeted inactivation of ppap2b in endothelial and hematopoietic cells display enhanced vascular inflammation and permeability.Arterioscler Thromb Vasc Biol. 2014 Apr;34(4):837-45. doi: 10.1161/ATVBAHA.113.302335. Epub 2014 Feb 6. Arterioscler Thromb Vasc Biol. 2014. PMID: 24504738 Free PMC article.
-
FTO-dependent m6A modification of Plpp3 in circSCMH1-regulated vascular repair and functional recovery following stroke.Nat Commun. 2023 Jan 30;14(1):489. doi: 10.1038/s41467-023-36008-y. Nat Commun. 2023. PMID: 36717587 Free PMC article.
-
Downregulation of Lipid Phosphate Phosphatase 3 Correlates With Tumor-Infiltrating Immune Cells in Oral Cancer.Cureus. 2022 Mar 27;14(3):e23553. doi: 10.7759/cureus.23553. eCollection 2022 Mar. Cureus. 2022. PMID: 35494957 Free PMC article.
References
-
- Sato Y, Kanno S, Oda N, Abe M, Ito M, Shitara K, Shibuya M. Properties of two VEGF receptors, Flt-1 and KDR, in signal transduction. Ann N Y Acad Sci. 2000;902:201–205. - PubMed
LinkOut - more resources
Full Text Sources

