ADAR1 interacts with NF90 through double-stranded RNA and regulates NF90-mediated gene expression independently of RNA editing
- PMID: 16055709
- PMCID: PMC1190226
- DOI: 10.1128/MCB.25.16.6956-6963.2005
ADAR1 interacts with NF90 through double-stranded RNA and regulates NF90-mediated gene expression independently of RNA editing
Abstract
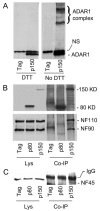
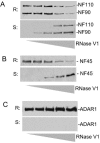
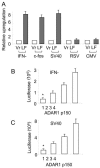
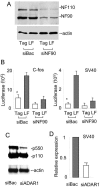
The RNA-editing enzyme ADAR1 modifies adenosines by deamination and produces A-to-I mutations in mRNA. ADAR1 was recently demonstrated to function in host defense and in embryonic erythropoiesis during fetal liver development. The mechanisms for these phenotypic effects are not yet known. Here we report a novel function of ADAR1 in the regulation of gene expression by interacting with the nuclear factor 90 (NF90) proteins, known regulators that bind the antigen response recognition element (ARRE-2) and have been demonstrated to stimulate transcription and translation. ADAR1 upregulates NF90-mediated gene expression by interacting with the NF90 proteins, including NF110, NF90, and NF45. A knockdown of NF90 with small interfering RNA suppresses this function of ADAR1. Coimmunoprecipitation and double-stranded RNA (dsRNA) digestion demonstrate that ADAR1 is associated with NF110, NF90, and NF45 through the bridge of cellular dsRNA. Studies with ADAR1 deletions demonstrate that the dsRNA binding domain and a region covering the Z-DNA binding domain and the nuclear export signal comprise the complete function of ADAR1 in upregulating NF90-mediated gene expression. These data suggest that ADAR1 has the potential both to change information content through editing of mRNA and to regulate gene expression through interacting with the NF90 family proteins.
Figures

Similar articles
-
ADAR1 interaction with Z-RNA promotes editing of endogenous double-stranded RNA and prevents MDA5-dependent immune activation.Cell Rep. 2021 Aug 10;36(6):109500. doi: 10.1016/j.celrep.2021.109500. Cell Rep. 2021. PMID: 34380029
-
Double-stranded RNA deaminase ADAR1 increases host susceptibility to virus infection.J Virol. 2007 Jan;81(2):917-23. doi: 10.1128/JVI.01527-06. Epub 2006 Nov 1. J Virol. 2007. PMID: 17079286 Free PMC article.
-
Induction of protein translation by ADAR1 within living cell nuclei is not dependent on RNA editing.Mol Cell. 2002 Nov;10(5):1235-46. doi: 10.1016/s1097-2765(02)00737-2. Mol Cell. 2002. PMID: 12453429
-
An RNA editor, adenosine deaminase acting on double-stranded RNA (ADAR1).J Interferon Cytokine Res. 2014 Jun;34(6):437-46. doi: 10.1089/jir.2014.0001. J Interferon Cytokine Res. 2014. PMID: 24905200 Free PMC article. Review.
-
Deciphering the Biological Significance of ADAR1-Z-RNA Interactions.Int J Mol Sci. 2021 Oct 23;22(21):11435. doi: 10.3390/ijms222111435. Int J Mol Sci. 2021. PMID: 34768866 Free PMC article. Review.
Cited by
-
The role of ADAR1 through and beyond its editing activity in cancer.Cell Commun Signal. 2024 Jan 17;22(1):42. doi: 10.1186/s12964-023-01465-x. Cell Commun Signal. 2024. PMID: 38233935 Free PMC article. Review.
-
ADAR enzyme and miRNA story: a nucleotide that can make the difference.Int J Mol Sci. 2013 Nov 19;14(11):22796-816. doi: 10.3390/ijms141122796. Int J Mol Sci. 2013. PMID: 24256817 Free PMC article. Review.
-
ADAR1 promotes the epithelial-to-mesenchymal transition and stem-like cell phenotype of oral cancer by facilitating oncogenic microRNA maturation.J Exp Clin Cancer Res. 2019 Jul 17;38(1):315. doi: 10.1186/s13046-019-1300-2. J Exp Clin Cancer Res. 2019. PMID: 31315644 Free PMC article.
-
Decreased A-to-I RNA editing as a source of keratinocytes' dsRNA in psoriasis.RNA. 2018 Jun;24(6):828-840. doi: 10.1261/rna.064659.117. Epub 2018 Mar 28. RNA. 2018. PMID: 29592874 Free PMC article.
-
NF45 and NF90 Bind HIV-1 RNA and Modulate HIV Gene Expression.Viruses. 2016 Feb 16;8(2):47. doi: 10.3390/v8020047. Viruses. 2016. PMID: 26891316 Free PMC article.
References
-
- Cattaneo, R. 1994. Biased (A 3 I) hypermutation of animal RNA virus genomes. Curr. Opin. Genet. Dev. 4:895-900. - PubMed
-
- Cho, D. S., W. Yang, J. T. Lee, R. Shiekhattar, J. M. Murray, and K. Nishikura. 2003. Requirement of dimerization for RNA editing activity of adenosine deaminases acting on RNA. J. Biol. Chem. 278:17093-17102. - PubMed
-
- Du, J., Y. Pan, Y. Shi, C. Guo, X. Jin, L. Sun, N. Liu, T. Qiao, and D. Fan. 2005. Overexpression and significance of prion protein in gastric cancer and multidrug-resistant gastric carcinoma cell line SGC7901/ADR. Int. J. Cancer 113:213-220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
