Helicobacter pylori VacA cytotoxin: a probe for a clathrin-independent and Cdc42-dependent pinocytic pathway routed to late endosomes
- PMID: 16055501
- PMCID: PMC1237088
- DOI: 10.1091/mbc.e05-05-0398
Helicobacter pylori VacA cytotoxin: a probe for a clathrin-independent and Cdc42-dependent pinocytic pathway routed to late endosomes
Abstract
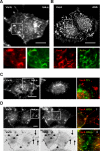
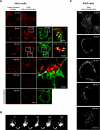
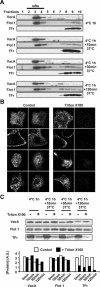
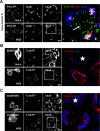
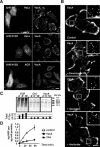
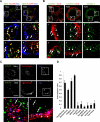
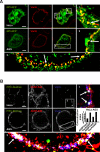
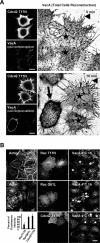
The vacuolating cytotoxin VacA is a major virulence factor of Helicobacter pylori, a bacterium responsible for gastroduodenal ulcers and cancer. VacA associates with lipid rafts, is endocytosed, and reaches the late endocytic compartment where it induces vacuolation. We have investigated the endocytic and intracellular trafficking pathways used by VacA, in HeLa and gastric AGS cells. We report here that VacA was first bound to plasma-membrane domains localized above F-actin structures that were controlled by the Rac1 GTPase. VacA was subsequently pinocytosed by a clathrin-independent mechanism into cell peripheral early endocytic compartments lacking caveolin 1, the Rab5 effector early endosomes antigen-1 (EEA1) and transferrin. These compartments took up fluid-phase (as evidenced by the accumulation of fluorescent dextran) and glycosylphosphatidylinositol-anchored proteins (GPI-APs). VacA pinocytosis was controlled by Cdc42 and did not require cellular tyrosine kinases, dynamin 2, ADP-ribosylating factor 6, or RhoA GTPase activities. VacA was subsequently routed to EEA1-sorting endosomes and then sorted to late endosomes. During all these different endocytic steps, VacA was continuously associated with detergent resistant membrane domains. From these results we propose that VacA might be a valuable probe to study raft-associated molecules, pinocytosed by a clathrin-independent mechanism, and routed to the degradative compartment.
Figures

Similar articles
-
GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway.Dev Cell. 2002 Apr;2(4):411-23. doi: 10.1016/s1534-5807(02)00145-4. Dev Cell. 2002. PMID: 11970892
-
Early endosomes associated with dynamic F-actin structures are required for late trafficking of H. pylori VacA toxin.J Cell Biol. 2007 Apr 23;177(2):343-54. doi: 10.1083/jcb.200609061. Epub 2007 Apr 16. J Cell Biol. 2007. PMID: 17438076 Free PMC article.
-
Arf6-independent GPI-anchored protein-enriched early endosomal compartments fuse with sorting endosomes via a Rab5/phosphatidylinositol-3'-kinase-dependent machinery.Mol Biol Cell. 2006 Aug;17(8):3689-704. doi: 10.1091/mbc.e05-10-0980. Epub 2006 Jun 7. Mol Biol Cell. 2006. PMID: 16760436 Free PMC article.
-
Vacuolating cytotoxin A (VacA) - A multi-talented pore-forming toxin from Helicobacter pylori.Toxicon. 2016 Aug;118:27-35. doi: 10.1016/j.toxicon.2016.04.037. Epub 2016 Apr 20. Toxicon. 2016. PMID: 27105670 Review.
-
Action site and cellular effects of cytotoxin VacA produced by Helicobacter pylori.Folia Microbiol (Praha). 1998;43(3):279-84. doi: 10.1007/BF02818613. Folia Microbiol (Praha). 1998. PMID: 9717255 Review.
Cited by
-
Remodeling the host environment: modulation of the gastric epithelium by the Helicobacter pylori vacuolating toxin (VacA).Front Cell Infect Microbiol. 2012 Mar 27;2:37. doi: 10.3389/fcimb.2012.00037. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 22919629 Free PMC article. Review.
-
Role of connexin 43 in Helicobacter pylori VacA-induced cell death.Infect Immun. 2014 Jan;82(1):423-32. doi: 10.1128/IAI.00827-13. Epub 2013 Nov 4. Infect Immun. 2014. PMID: 24191302 Free PMC article.
-
Molecular cross-talk between Helicobacter pylori and human gastric mucosa.World J Gastroenterol. 2011 Mar 21;17(11):1383-99. doi: 10.3748/wjg.v17.i11.1383. World J Gastroenterol. 2011. PMID: 21472096 Free PMC article. Review.
-
Distinct mechanisms of clathrin-independent endocytosis have unique sphingolipid requirements.Mol Biol Cell. 2006 Jul;17(7):3197-210. doi: 10.1091/mbc.e05-12-1101. Epub 2006 May 3. Mol Biol Cell. 2006. PMID: 16672382 Free PMC article.
-
Protein transduction in human cells is enhanced by cell-penetrating peptides fused with an endosomolytic HA2 sequence.Peptides. 2012 Oct;37(2):273-84. doi: 10.1016/j.peptides.2012.07.019. Epub 2012 Jul 31. Peptides. 2012. PMID: 22898256 Free PMC article.
References
-
- Adair, W. L., and Kornfeld, S. (1974). Isolation of the receptors for wheat germ agglutinin and the Ricinus communis lectins from human erythrocytes using affinity chromatography. J. Biol. Chem. 249, 4696-4704.
-
- Benmerah, A., Bayrou, M., Cerf-Bensussan, N., and Dautry-Varsat, A. (1999). Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112, 1303-1311. - PubMed
-
- Blanke, S. R. (2005). Micro-managing the executioner: pathogen targeting of mitochondria. Trends Microbiol. 13, 64-71. - PubMed
-
- Boquet, P., and Lemichez, E. (2003). Bacterial virulence factors targeting Rho GTPases: parasitism or symbiosis? Trends Cell Biol. 13, 238-246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

