Involvement of the portal at an early step in herpes simplex virus capsid assembly
- PMID: 16051846
- PMCID: PMC1182615
- DOI: 10.1128/JVI.79.16.10540-10546.2005
Involvement of the portal at an early step in herpes simplex virus capsid assembly
Abstract
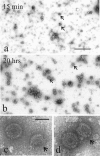
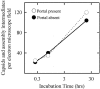
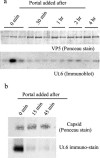
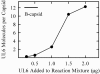
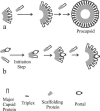
DNA enters the herpes simplex virus capsid by way of a ring-shaped structure called the portal. Each capsid contains a single portal, located at a unique capsid vertex, that is composed of 12 UL6 protein molecules. The position of the portal requires that capsid formation take place in such a way that a portal is incorporated into one of the 12 capsid vertices and excluded from all other locations, including the remaining 11 vertices. Since initiation or nucleation of capsid formation is a unique step in the overall assembly process, involvement of the portal in initiation has the potential to cause its incorporation into a unique vertex. In such a mode of assembly, the portal would need to be involved in initiation but not able to be inserted in subsequent assembly steps. We have used an in vitro capsid assembly system to test whether the portal is involved selectively in initiation. Portal incorporation was compared in capsids assembled from reactions in which (i) portals were present at the beginning of the assembly process and (ii) portals were added after assembly was under way. The results showed that portal-containing capsids were formed only if portals were present at the outset of assembly. A delay caused formation of capsids lacking portals. The findings indicate that if portals are present in reaction mixtures, a portal is incorporated during initiation or another early step in assembly. If no portals are present, assembly is initiated in another, possibly related, way that does not involve a portal.
Figures

Similar articles
-
Assembly of the herpes simplex virus capsid: identification of soluble scaffold-portal complexes and their role in formation of portal-containing capsids.J Virol. 2003 Sep;77(18):9862-71. doi: 10.1128/jvi.77.18.9862-9871.2003. J Virol. 2003. PMID: 12941896 Free PMC article.
-
The UL6 gene product forms the portal for entry of DNA into the herpes simplex virus capsid.J Virol. 2001 Nov;75(22):10923-32. doi: 10.1128/JVI.75.22.10923-10932.2001. J Virol. 2001. PMID: 11602732 Free PMC article.
-
Identification of a region in the herpes simplex virus scaffolding protein required for interaction with the portal.J Virol. 2005 Jan;79(1):132-9. doi: 10.1128/JVI.79.1.132-139.2005. J Virol. 2005. PMID: 15596809 Free PMC article.
-
Herpesvirus capsid assembly: insights from structural analysis.Curr Opin Virol. 2011 Aug;1(2):142-9. doi: 10.1016/j.coviro.2011.06.003. Curr Opin Virol. 2011. PMID: 21927635 Free PMC article. Review.
-
Portal Protein: The Orchestrator of Capsid Assembly for the dsDNA Tailed Bacteriophages and Herpesviruses.Annu Rev Virol. 2019 Sep 29;6(1):141-160. doi: 10.1146/annurev-virology-092818-015819. Epub 2019 Jul 23. Annu Rev Virol. 2019. PMID: 31337287 Free PMC article. Review.
Cited by
-
Uncoupling uncoating of herpes simplex virus genomes from their nuclear import and gene expression.J Virol. 2011 May;85(9):4271-83. doi: 10.1128/JVI.02067-10. Epub 2011 Feb 23. J Virol. 2011. PMID: 21345968 Free PMC article.
-
Amino acids 143 to 150 of the herpes simplex virus type 1 scaffold protein are required for the formation of portal-containing capsids.J Virol. 2008 Jul;82(13):6778-81. doi: 10.1128/JVI.00473-08. Epub 2008 Apr 16. J Virol. 2008. PMID: 18417585 Free PMC article.
-
Capsids and Portals Influence Each Other's Conformation During Assembly and Maturation.J Mol Biol. 2020 Mar 27;432(7):2015-2029. doi: 10.1016/j.jmb.2020.01.022. Epub 2020 Feb 6. J Mol Biol. 2020. PMID: 32035900 Free PMC article.
-
Disulfide bond formation in the herpes simplex virus 1 UL6 protein is required for portal ring formation and genome encapsidation.J Virol. 2011 Sep;85(17):8616-24. doi: 10.1128/JVI.00123-11. Epub 2011 May 18. J Virol. 2011. PMID: 21593161 Free PMC article.
-
Cryo-electron tomography of bacteriophage phi6 procapsids shows random occupancy of the binding sites for RNA polymerase and packaging NTPase.J Struct Biol. 2010 Sep;171(3):389-96. doi: 10.1016/j.jsb.2010.06.005. Epub 2010 Jun 9. J Struct Biol. 2010. PMID: 20538059 Free PMC article.
References
-
- Bazinet, C., and J. King. 1988. Initiation of P22 procapsid assembly in vivo. J. Mol. Biol. 202:77-86. - PubMed
-
- Droge, A., M. A. Santos, A. C. Stiege, J. C. Alonso, R. Lurz, T. A. Trautner, and P. Tavares. 2000. Shape and DNA packaging activity of bacteriophage SPP1 procapsid: protein components and interactions during assembly. J. Mol. Biol. 296:117-132. - PubMed
-
- Heymann, J. B., N. Cheng, W. W. Newcomb, B. L. Trus, J. C. Brown, and A. C. Steven. 2003. Dynamics of herpes simplex virus capsid maturation visualized by time-lapse cryo-electron microscopy. Nat. Struct. Biol. 10:334-341. - PubMed
-
- Homa, F. L., and J. C. Brown. 1997. Capsid assembly and DNA packaging in herpes simplex virus. Rev. Med. Virol. 7:107-122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

