LSECtin interacts with filovirus glycoproteins and the spike protein of SARS coronavirus
- PMID: 16051304
- PMCID: PMC7111772
- DOI: 10.1016/j.virol.2005.06.026
LSECtin interacts with filovirus glycoproteins and the spike protein of SARS coronavirus
Abstract
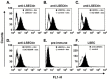
Cellular attachment factors like the C-type lectins DC-SIGN and DC-SIGNR (collectively referred to as DC-SIGN/R) can augment viral infection and might promote viral dissemination in and between hosts. The lectin LSECtin is encoded in the same chromosomal locus as DC-SIGN/R and is coexpressed with DC-SIGNR on sinusoidal endothelial cells in liver and lymphnodes. Here, we show that LSECtin enhances infection driven by filovirus glycoproteins (GP) and the S protein of SARS coronavirus, but does not interact with human immunodeficiency virus type-1 and hepatitis C virus envelope proteins. Ligand binding to LSECtin was inhibited by EGTA but not by mannan, suggesting that LSECtin unlike DC-SIGN/R does not recognize high-mannose glycans on viral GPs. Finally, we demonstrate that LSECtin is N-linked glycosylated and that glycosylation is required for cell surface expression. In summary, we identified LSECtin as an attachment factor that in conjunction with DC-SIGNR might concentrate viral pathogens in liver and lymph nodes.
Figures
Similar articles
-
Interactions of LSECtin and DC-SIGN/DC-SIGNR with viral ligands: Differential pH dependence, internalization and virion binding.Virology. 2008 Mar 30;373(1):189-201. doi: 10.1016/j.virol.2007.11.001. Epub 2008 Feb 20. Virology. 2008. PMID: 18083206 Free PMC article.
-
DC-SIGN and DC-SIGNR interact with the glycoprotein of Marburg virus and the S protein of severe acute respiratory syndrome coronavirus.J Virol. 2004 Nov;78(21):12090-5. doi: 10.1128/JVI.78.21.12090-12095.2004. J Virol. 2004. PMID: 15479853 Free PMC article.
-
C-type lectin LSECtin interacts with DC-SIGNR and is involved in hepatitis C virus binding.Mol Cell Biochem. 2009 Jul;327(1-2):183-90. doi: 10.1007/s11010-009-0056-y. Epub 2009 Feb 21. Mol Cell Biochem. 2009. PMID: 19234677 Free PMC article.
-
DC-SIGN, DC-SIGNR and LSECtin: C-type lectins for infection.Int Rev Immunol. 2014 Jan;33(1):54-66. doi: 10.3109/08830185.2013.834897. Epub 2013 Oct 24. Int Rev Immunol. 2014. PMID: 24156700 Review.
-
The role of DC-SIGN and DC-SIGNR in HIV and Ebola virus infection: can potential therapeutics block virus transmission and dissemination?Expert Opin Ther Targets. 2002 Aug;6(4):423-31. doi: 10.1517/14728222.6.4.423. Expert Opin Ther Targets. 2002. PMID: 12223058 Review.
Cited by
-
Characterization and Expression Analysis of the C-Type Lectin Ladderlectin in Litopenaeus vannamei Post-WSSV Infection.Biology (Basel). 2024 Sep 24;13(10):758. doi: 10.3390/biology13100758. Biology (Basel). 2024. PMID: 39452067 Free PMC article.
-
Unprecedented selectivity for homologous lectin targets: differential targeting of the viral receptors L-SIGN and DC-SIGN.Chem Sci. 2024 Aug 27;15(37):15352-66. doi: 10.1039/d4sc02980a. Online ahead of print. Chem Sci. 2024. PMID: 39246372 Free PMC article.
-
Severe COVID-19 infection: An institutional review and literature overview.PLoS One. 2024 Aug 20;19(8):e0304960. doi: 10.1371/journal.pone.0304960. eCollection 2024. PLoS One. 2024. PMID: 39163410 Free PMC article. Review.
-
Refined innate plasma signature after rVSVΔG-ZEBOV-GP immunization is shared among adult cohorts in Europe and North America.Front Immunol. 2024 Jan 3;14:1279003. doi: 10.3389/fimmu.2023.1279003. eCollection 2023. Front Immunol. 2024. PMID: 38235127 Free PMC article.
-
Infection of liver sinusoidal endothelial cells with Muromegalovirus muridbeta1 involves binding to neuropilin-1 and is dynamin-dependent.Front Cell Infect Microbiol. 2023 Nov 9;13:1249894. doi: 10.3389/fcimb.2023.1249894. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38029264 Free PMC article.
References
-
- Appelmelk B.J., van D.I., Van Vliet S.J., Vandenbroucke-Grauls C.M., Geijtenbeek T.B., Van Kooyk Y. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J. Immunol. 2003;170:1635–1639. - PubMed
-
- Baribaud F., Doms R.W., Pohlmann S. The role of DC-SIGN and DC-SIGNR in HIV and Ebola virus infection: can potential therapeutics block virus transmission and dissemination? Expert Opin. Ther. Targets. 2002;6:423–431. - PubMed
-
- Bashirova A.A., Geijtenbeek T.B., van Duijnhoven G.C., Van Vliet S.J., Eilering J.B., Martin M.P., Wu L., Martin T.D., Viebig N., Knolle P.A., KewalRamani V.N., Van Kooyk Y., Carrington M. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 2001;193:671–678. - PMC - PubMed
-
- Becker S., Spiess M., Klenk H.D. The asialoglycoprotein receptor is a potential liver-specific receptor for Marburg virus. J. Gen. Virol. 1995;76(Pt. 2):393–399. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

