Evaluation of inapparent nosocomial severe acute respiratory syndrome coronavirus infection in Vietnam by use of highly specific recombinant truncated nucleocapsid protein-based enzyme-linked immunosorbent assay
- PMID: 16002634
- PMCID: PMC1182204
- DOI: 10.1128/CDLI.12.7.848-854.2005
Evaluation of inapparent nosocomial severe acute respiratory syndrome coronavirus infection in Vietnam by use of highly specific recombinant truncated nucleocapsid protein-based enzyme-linked immunosorbent assay
Abstract
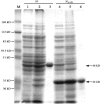
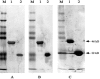
Severe acute respiratory syndrome (SARS) is a recently emerged human disease associated with pneumonia. Inapparent infection with SARS coronavirus (CoV) is not well characterized. To develop a safe, simple, and reliable screening method for SARS diagnosis and epidemiological study, two recombinant SARS-CoV nucleocapsid proteins (N' protein and (N)Delta(121) protein) were expressed in Escherichia coli, purified by affinity chromatography, and used as antigens for indirect, immunoglobulin G enzyme-linked immunosorbent assays (ELISA). Serum samples collected from healthy volunteers and SARS patients in Vietnam were used to evaluate the newly developed methods. The N' protein-based ELISA showed a highly nonspecific reaction. The (N)Delta(121) protein-based ELISA, with a nonspecific reaction drastically reduced compared to that of the nearly-whole-length N' protein-based ELISA, resulted in higher rates of positive reactions, higher titers, and earlier detection than the SARS-CoV-infected cell lysate-based ELISA. These results indicate that our newly developed SARS-CoV (N)Delta(121) protein-based ELISA is not only safe but also a more specific and more sensitive method to diagnose SARS-CoV infection and hence a useful tool for large-scale epidemiological studies. To identify inapparent SARS-CoV infections, serum samples collected from health care workers (HCWs) in Vietnam were screened by the (N)Delta(121) protein-based ELISA, and positive samples were confirmed by a virus neutralization test. Four out of 149 HCWs were identified to have inapparent SARS-CoV infection in Vietnam, indicating that subclinical SARS-CoV infection in Vietnam is rare but does exist.
Figures

Similar articles
-
Evaluation of antibody responses against SARS coronaviral nucleocapsid or spike proteins by immunoblotting or ELISA.J Med Virol. 2004 Jul;73(3):338-46. doi: 10.1002/jmv.20096. J Med Virol. 2004. PMID: 15170626 Free PMC article.
-
Longitudinal profile of immunoglobulin G (IgG), IgM, and IgA antibodies against the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in patients with pneumonia due to the SARS coronavirus.Clin Diagn Lab Immunol. 2004 Jul;11(4):665-8. doi: 10.1128/CDLI.11.4.665-668.2004. Clin Diagn Lab Immunol. 2004. PMID: 15242938 Free PMC article.
-
Relative rates of non-pneumonic SARS coronavirus infection and SARS coronavirus pneumonia.Lancet. 2004 Mar 13;363(9412):841-5. doi: 10.1016/S0140-6736(04)15729-2. Lancet. 2004. PMID: 15031027 Free PMC article.
-
Understanding the accessory viral proteins unique to the severe acute respiratory syndrome (SARS) coronavirus.Antiviral Res. 2006 Nov;72(2):78-88. doi: 10.1016/j.antiviral.2006.05.010. Epub 2006 Jun 6. Antiviral Res. 2006. PMID: 16820226 Free PMC article. Review.
-
Severe acute respiratory syndrome (SARS): development of diagnostics and antivirals.Ann N Y Acad Sci. 2006 May;1067(1):500-5. doi: 10.1196/annals.1354.072. Ann N Y Acad Sci. 2006. PMID: 16804033 Free PMC article. Review.
Cited by
-
A highly sensitive nanobody-based immunoassay detecting SARS-CoV-2 nucleocapsid protein using all-recombinant reagents.Front Immunol. 2023 Jul 11;14:1220477. doi: 10.3389/fimmu.2023.1220477. eCollection 2023. Front Immunol. 2023. PMID: 37497229 Free PMC article.
-
Identification of B-Cell Linear Epitopes in the Nucleocapsid (N) Protein B-Cell Linear Epitopes Conserved among the Main SARS-CoV-2 Variants.Viruses. 2023 Apr 6;15(4):923. doi: 10.3390/v15040923. Viruses. 2023. PMID: 37112903 Free PMC article.
-
A new multiplex SARS-CoV-2 antigen microarray showed correlation of IgG, IgA, and IgM antibodies from patients with COVID-19 disease severity and maintenance of relative IgA and IgM antigen binding over time.PLoS One. 2023 Mar 30;18(3):e0283537. doi: 10.1371/journal.pone.0283537. eCollection 2023. PLoS One. 2023. PMID: 36996259 Free PMC article.
-
Recent advances in immunoassay technologies for the detection of human coronavirus infections.Front Cell Infect Microbiol. 2023 Jan 4;12:1040248. doi: 10.3389/fcimb.2022.1040248. eCollection 2022. Front Cell Infect Microbiol. 2023. PMID: 36683684 Free PMC article. Review.
-
Fcγ-Receptor-Based Enzyme-Linked Immunosorbent Assays for Sensitive, Specific, and Persistent Detection of Anti-SARS-CoV-2 Nucleocapsid Protein IgG Antibodies in Human Sera.J Clin Microbiol. 2022 Jun 15;60(6):e0007522. doi: 10.1128/jcm.00075-22. Epub 2022 May 16. J Clin Microbiol. 2022. PMID: 35574677 Free PMC article.
References
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
-
- Centers for Disease Control and Prevention. 2003. Outbreak of acute respiratory syndrome worldwide, 2003. Morb. Mortal. Wkly. Rep. 52:226-228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

