Contributions of the viral genetic background and a single amino acid substitution in an immunodominant CD8+ T-cell epitope to murine coronavirus neurovirulence
- PMID: 15994805
- PMCID: PMC1168726
- DOI: 10.1128/JVI.79.14.9108-9118.2005
Contributions of the viral genetic background and a single amino acid substitution in an immunodominant CD8+ T-cell epitope to murine coronavirus neurovirulence
Abstract
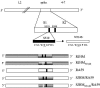
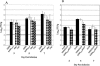
The immunodominant CD8+ T-cell epitope of a highly neurovirulent strain of mouse hepatitis virus (MHV), JHM, is thought to be essential for protection against virus persistence within the central nervous system. To test whether abrogation of this H-2Db-restricted epitope, located within the spike glycoprotein at residues S510 to 518 (S510), resulted in delayed virus clearance and/or virus persistence we selected isogenic recombinants which express either the wild-type JHM spike protein (RJHM) or spike containing the N514S mutation (RJHM(N514S)), which abrogates the response to S510. In contrast to observations in suckling mice in which viruses encoding inactivating mutations within the S510 epitope (epitope escape mutants) were associated with persistent virus and increased neurovirulence (Pewe et al., J Virol. 72:5912-5918, 1998), RJHM(N514S) was not more virulent than the parental, RJHM, in 4-week-old C57BL/6 (H-2b) mice after intracranial injection. Recombinant viruses expressing the JHM spike, wild type or encoding the N514S substitution, were also selected in which background genes were derived from the neuroattenuated A59 strain of MHV. Whereas recombinants expressing the wild-type JHM spike (SJHM/RA59) were highly neurovirulent, A59 recombinants containing the N514S mutation (SJHM(N514S)/RA59) were attenuated, replicated less efficiently, and exhibited reduced virus spread in the brain at 5 days postinfection (peak of infectious virus titers in the central nervous system) compared to parental virus encoding wild-type spike. Virulence assays in BALB/c mice (H-2d), which do not recognize the S510 epitope, revealed that attenuation of the epitope escape mutants was not due to the loss of a pathogenic immune response directed against the S510 epitope. Thus, an intact immunodominant S510 epitope is not essential for virus clearance from the CNS, the S510 inactivating mutation results in decreased virulence in weanling mice but not in suckling mice, suggesting that specific host conditions are required for epitope escape mutants to display increased virulence, and the N514S mutation causes increased attenuation in the context of A59 background genes, demonstrating that genes other than that for the spike are also important in determining neurovirulence.
Figures



Similar articles
-
The spike glycoprotein of murine coronavirus MHV-JHM mediates receptor-independent infection and spread in the central nervous systems of Ceacam1a-/- Mice.J Virol. 2008 Jan;82(2):755-63. doi: 10.1128/JVI.01851-07. Epub 2007 Nov 14. J Virol. 2008. PMID: 18003729 Free PMC article.
-
Effects of an epitope-specific CD8+ T-cell response on murine coronavirus central nervous system disease: protection from virus replication and antigen spread and selection of epitope escape mutants.J Virol. 2004 Feb;78(3):1150-9. doi: 10.1128/jvi.78.3.1150-1159.2004. J Virol. 2004. PMID: 14722270 Free PMC article.
-
Priming of CD8+ T cells during central nervous system infection with a murine coronavirus is strain dependent.J Virol. 2008 Jul;82(13):6150-60. doi: 10.1128/JVI.00106-08. Epub 2008 Apr 16. J Virol. 2008. PMID: 18417581 Free PMC article.
-
Murine coronavirus neuropathogenesis: determinants of virulence.J Neurovirol. 2010 Nov;16(6):427-34. doi: 10.3109/13550284.2010.529238. Epub 2010 Nov 12. J Neurovirol. 2010. PMID: 21073281 Free PMC article. Review.
-
Pathogenesis of acute and chronic central nervous system infection with variants of mouse hepatitis virus, strain JHM.Immunol Res. 2007;39(1-3):160-72. doi: 10.1007/s12026-007-0079-y. Immunol Res. 2007. PMID: 17917063 Free PMC article. Review.
Cited by
-
Neurological manifestations of coronavirus infections - a systematic review.Ann Clin Transl Neurol. 2020 Oct;7(10):2057-2071. doi: 10.1002/acn3.51166. Epub 2020 Aug 27. Ann Clin Transl Neurol. 2020. PMID: 32853453 Free PMC article.
-
A hypervariable region within the 3' cis-acting element of the murine coronavirus genome is nonessential for RNA synthesis but affects pathogenesis.J Virol. 2007 Feb;81(3):1274-87. doi: 10.1128/JVI.00803-06. Epub 2006 Nov 8. J Virol. 2007. PMID: 17093194 Free PMC article.
-
Murine coronavirus receptors are differentially expressed in the central nervous system and play virus strain-dependent roles in neuronal spread.J Virol. 2010 Nov;84(21):11030-44. doi: 10.1128/JVI.02688-09. Epub 2010 Aug 25. J Virol. 2010. PMID: 20739537 Free PMC article.
-
The murine coronavirus nucleocapsid gene is a determinant of virulence.J Virol. 2010 Feb;84(4):1752-63. doi: 10.1128/JVI.01758-09. Epub 2009 Dec 9. J Virol. 2010. PMID: 20007284 Free PMC article.
-
Non-invasive imaging of mouse hepatitis coronavirus infection reveals determinants of viral replication and spread in vivo.Cell Microbiol. 2009 May;11(5):825-41. doi: 10.1111/j.1462-5822.2009.01298.x. Epub 2009 Feb 10. Cell Microbiol. 2009. PMID: 19215224 Free PMC article.
References
-
- Barouch, D. H., J. Kunstman, J. Glowczwskie, K. J. Kunstman, M. A. Egan, F. W. Peyerl, S. Santra, M. J. Kuroda, J. E. Schmitz, K. Beaudry, G. R. Krivulka, M. A. Lifton, D. A. Gorgone, S. M. Wolinsky, and N. L. Letvin. 2003. Viral escape from dominant simian immunodeficiency virus epitope-specific cytotoxic T lymphocytes in DNA-vaccinated rhesus monkeys. J. Virol. 77:7367-7375. - PMC - PubMed
-
- Bergmann, C. C., J. D. Altman, D. Hinton, and S. A. Stohlman. 1999. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 163:3379-3387. - PubMed
-
- Bergmann, C. C., Q. Yao, M. Lin, and S. A. Stohlman. 1996. The JHM strain of mouse hepatitis virus induces a spike protein-specific Db-restricted cytotoxic T-cell response. J. Gen. Virol. 77:315-325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

