C-terminal domain of hepatitis C virus core protein is essential for secretion
- PMID: 15991288
- PMCID: PMC4504891
- DOI: 10.3748/wjg.v11.i25.3887
C-terminal domain of hepatitis C virus core protein is essential for secretion
Abstract
Aim: We have previously demonstrated that hepatitis C virus (HCV) core protein is efficiently released into the culture medium in insect cells. The objective of this study is to characterize the HCV core secretion in insect cells.
Methods: We constructed recombinant baculoviruses expressing various-length of mutant core proteins, expressed these proteins in insect cells, and examined core protein secretion in insect cells.
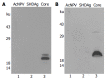
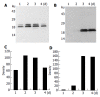
Results: Only wild type core was efficiently released into the culture medium, although the protein expression level of wild type core was lower than those of other mutant core proteins. We found that the shorter form of the core construct expressed the higher level of protein. However, if more than 18 amino acids of the core were truncated at the C-terminus, core proteins were no longer secreted into the culture medium. Membrane flotation data show that the secreted core proteins are associated with the cellular membrane protein, indicating that HCV core is secreted as a membrane complex.
Conclusion: The C-terminal 18 amino acids of HCV core were crucial for core secretion into the culture media. Since HCV replication occurs on lipid raft membrane structure, these results suggest that HCV may utilize a unique core release mechanism to escape immune surveillance, thereby potentially representing the feature of HCV morphogenesis.
Figures



Similar articles
-
Hepatitis C virus core protein is efficiently released into the culture medium in insect cells.J Biochem Mol Biol. 2004 Nov 30;37(6):735-40. doi: 10.5483/bmbrep.2004.37.6.735. J Biochem Mol Biol. 2004. PMID: 15607034
-
Mechanisms for inhibition of hepatitis B virus gene expression and replication by hepatitis C virus core protein.J Biol Chem. 2003 Jan 3;278(1):591-607. doi: 10.1074/jbc.M204241200. Epub 2002 Oct 24. J Biol Chem. 2003. PMID: 12401801
-
[Characterization of hepatitis C virus structural proteins and HCV-like particles produced in recombinant baculovirus infected insect cells].Mol Biol (Mosk). 2010 Jan-Feb;44(1):107-19. Mol Biol (Mosk). 2010. PMID: 20198865 Russian.
-
Development and characterisation of recombinant hepatitis delta virus-like particles.Virus Genes. 2001;23(1):97-104. doi: 10.1023/a:1011195715747. Virus Genes. 2001. PMID: 11556408
-
Signal peptide peptidase-catalyzed cleavage of hepatitis C virus core protein is dispensable for virus budding but destabilizes the viral capsid.J Biol Chem. 2006 Sep 22;281(38):27679-92. doi: 10.1074/jbc.M602587200. Epub 2006 Jul 18. J Biol Chem. 2006. PMID: 16849324
Cited by
-
Role of Capsid Anchor in the Morphogenesis of Zika Virus.J Virol. 2018 Oct 29;92(22):e01174-18. doi: 10.1128/JVI.01174-18. Print 2018 Nov 15. J Virol. 2018. PMID: 30158295 Free PMC article.
-
The Hepatitis E Virus Open Reading Frame 2 Protein: Beyond Viral Capsid.Front Microbiol. 2021 Oct 7;12:739124. doi: 10.3389/fmicb.2021.739124. eCollection 2021. Front Microbiol. 2021. PMID: 34690982 Free PMC article. Review.
References
-
- Alter HJ, Purcell RH, Shih JW, Melpolder JC, Houghton M, Choo QL, Kuo G. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989;321:1494–1500. - PubMed
-
- Kuo G, Choo QL, Alter HJ, Gitnick GL, Redeker AG, Purcell RH, Miyamura T, Dienstag JL, Alter MJ, Tegtmeier CE, et al. An assay for circulating anti-bodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989;244:362–364. - PubMed
-
- Shimotohno K. Hepatocellular carcinoma in Japan and its linkage to infection with hepatitis C virus. Semin Virol. 1993;4:305–312.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

