Integrin-mediated dendrite branch maintenance requires Abelson (Abl) family kinases
- PMID: 15987940
- PMCID: PMC6725048
- DOI: 10.1523/JNEUROSCI.1432-05.2005
Integrin-mediated dendrite branch maintenance requires Abelson (Abl) family kinases
Abstract
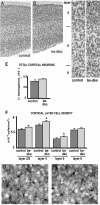
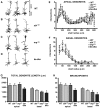
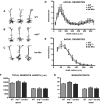
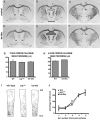
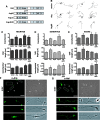
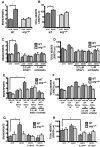
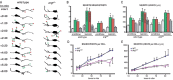
Dendrite arbor structure is a critical determinant of nervous system function that must be actively maintained throughout life, but the signaling pathways that regulate dendrite maintenance are essentially unknown. We report that the Abelson (Abl) and Abl-related gene (Arg) nonreceptor tyrosine kinases are required for maintenance of cortical dendrites in the mouse brain. arg-/- cortical dendrites initially develop normally and are indistinguishable from wild-type dendrites at postnatal day 21. Dendrite branches are not efficiently maintained in arg-/- neurons, leading to a reduction in dendrite arbor size by early adulthood. More severe dendrite loss is observed in abl-/-arg-/- neurons. Elevation of Arg kinase activity in primary cortical neurons promotes axon and dendrite branching. Activation of integrin receptors by adhesion to laminin-1 or Semaphorin 7A also promotes neurite branching in cortical neurons, but this response is absent in arg-/- neurons because of the reduced dynamic behavior of mutant neurite branches. These data suggest that integrin signaling through Abl and Arg support cortical dendrite branch maintenance by promoting dendrite branch dynamics in response to adhesive cues.
Figures
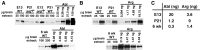
Similar articles
-
Arg kinase signaling in dendrite and synapse stabilization pathways: memory, cocaine sensitivity, and stress.Int J Biochem Cell Biol. 2013 Nov;45(11):2496-500. doi: 10.1016/j.biocel.2013.07.018. Epub 2013 Aug 2. Int J Biochem Cell Biol. 2013. PMID: 23916785 Free PMC article. Review.
-
The stimulation of dendrite growth by Sema3A requires integrin engagement and focal adhesion kinase.J Cell Sci. 2009 Jun 15;122(Pt 12):2034-42. doi: 10.1242/jcs.038232. Epub 2009 May 19. J Cell Sci. 2009. PMID: 19454481
-
Inhibition of Rho via Arg and p190RhoGAP in the postnatal mouse hippocampus regulates dendritic spine maturation, synapse and dendrite stability, and behavior.J Neurosci. 2007 Oct 10;27(41):10982-92. doi: 10.1523/JNEUROSCI.0793-07.2007. J Neurosci. 2007. PMID: 17928439 Free PMC article.
-
Loss of dendrite stabilization by the Abl-related gene (Arg) kinase regulates behavioral flexibility and sensitivity to cocaine.Proc Natl Acad Sci U S A. 2009 Sep 29;106(39):16859-64. doi: 10.1073/pnas.0902286106. Epub 2009 Sep 11. Proc Natl Acad Sci U S A. 2009. PMID: 19805386 Free PMC article.
-
Regulation of neuronal morphogenesis and synaptic function by Abl family kinases.Curr Opin Neurobiol. 2003 Oct;13(5):535-44. doi: 10.1016/j.conb.2003.08.002. Curr Opin Neurobiol. 2003. PMID: 14630215 Review.
Cited by
-
Glutamate Receptors and C-ABL Inhibitors: A New Therapeutic Approach for Parkinson's Disease.Cent Nerv Syst Agents Med Chem. 2024;24(1):22-44. doi: 10.2174/0118715249268627231206115942. Cent Nerv Syst Agents Med Chem. 2024. PMID: 38273763 Review.
-
Suppression of β1-integrin in gonadotropin-releasing hormone cells disrupts migration and axonal extension resulting in severe reproductive alterations.J Neurosci. 2012 Nov 21;32(47):16992-7002. doi: 10.1523/JNEUROSCI.3057-12.2012. J Neurosci. 2012. PMID: 23175850 Free PMC article.
-
Abl2 is recruited to ventral actin waves through cytoskeletal interactions to promote lamellipodium extension.Mol Biol Cell. 2018 Nov 15;29(23):2863-2873. doi: 10.1091/mbc.E18-01-0044. Epub 2018 Sep 26. Mol Biol Cell. 2018. PMID: 30256707 Free PMC article.
-
Emerging targets signaling for inflammation in Parkinson's disease drug discovery.Metab Brain Dis. 2022 Oct;37(7):2143-2161. doi: 10.1007/s11011-022-00999-2. Epub 2022 May 10. Metab Brain Dis. 2022. PMID: 35536461 Review.
-
Abelson phosphorylation of CLASP2 modulates its association with microtubules and actin.Cytoskeleton (Hoboken). 2014 Mar;71(3):195-209. doi: 10.1002/cm.21164. Epub 2014 Mar 12. Cytoskeleton (Hoboken). 2014. PMID: 24520051 Free PMC article.
References
-
- Anderson KL, Ferreira A (2004) alpha1 integrin activation: a link between beta-amyloid deposition and neuronal death in aging hippocampal neurons. J Neurosci Res 75: 688-697. - PubMed
-
- Bashaw GJ, Kidd T, Murray D, Pawson T, Goodman CS (2000) Repulsive axon guidance: abelson and enabled play opposing roles downstream of the roundabout receptor. Cell 101: 703-715. - PubMed
-
- Bi X, Lynch G, Zhou J, Gall CM (2001) Polarized distribution of alpha5 integrin in dendrites of hippocampal and cortical neurons. J Comp Neurol 435: 184-193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
