Protein tyrosine phosphatase kappa and SHP-1 are involved in the regulation of cell-cell contacts at adherens junctions in the exocrine pancreas
- PMID: 15987791
- PMCID: PMC1774702
- DOI: 10.1136/gut.2004.063164
Protein tyrosine phosphatase kappa and SHP-1 are involved in the regulation of cell-cell contacts at adherens junctions in the exocrine pancreas
Abstract
Background: We have previously shown that cell contacts between pancreatic acinar cells dissociate early in pancreatitis and that this is a prerequisite for the development of pancreatic oedema. Here we studied the underlying mechanism.
Methods: Employing experimental caerulein induced pancreatitis in vivo and isolated pancreatic acini ex vivo, in conjunction with protein chemistry, morphology, and electron microscopy, we determined whether cell contact regulation in the pancreas requires or involves: (1) changes in cadherin-catenin protein expression, (2) tyrosine phosphorylation of adhesion proteins, or (3) alterations in the actin cytoskeleton.
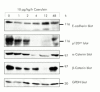
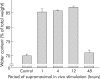
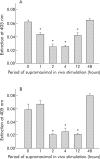
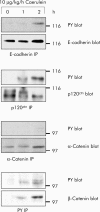
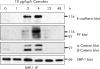
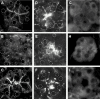
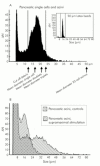
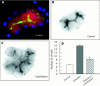
Results: During initial cell-cell contact dissociation at adherens junctions, expression of adhesion proteins remained stable. At time points of dissociated adherens junctions, the cadherin-catenin complex was found to be tyrosine phosphorylated and internalised. The receptor type protein tyrosine phosphatase (PTP)kappa was constitutively associated with the cadherin-catenin complex at intact cell contacts whereas following the dissociation of adherens junctions, the internalised components of the cadherin-catenin complex were tyrosine phosphorylated and associated with the cytosolic PTP SHP-1. In isolated acini, inhibition of endogenous protein tyrosine phosphatases alone was sufficient to induce dissociation of adherens junctions analogous to that found with supramaximal caerulein stimulation. Dissociation of actin microfilaments had no effect on adherens junction integrity.
Conclusions: These data identify tyrosine phosphorylation as the key regulator for cell contacts at adherens junctions and suggest a definitive role for the protein tyrosine phosphatases PTPkappa and SHP-1 in the regulation, maintenance, and restitution of cell adhesions in a complex epithelial organ such as the pancreas.
Figures


Comment in
-
The importance of keeping in touch: regulation of cell-cell contact in the exocrine pancreas.Gut. 2005 Oct;54(10):1358-9. doi: 10.1136/gut.2005.070953. Gut. 2005. PMID: 16162948 Free PMC article. No abstract available.
Similar articles
-
Beta-catenin interacts with low-molecular-weight protein tyrosine phosphatase leading to cadherin-mediated cell-cell adhesion increase.Cancer Res. 2002 Nov 15;62(22):6489-99. Cancer Res. 2002. PMID: 12438242
-
Regulation of VE-cadherin linkage to the cytoskeleton in endothelial cells exposed to fluid shear stress.Exp Cell Res. 2002 Feb 15;273(2):240-7. doi: 10.1006/excr.2001.5453. Exp Cell Res. 2002. PMID: 11822879
-
Turn-off, drop-out: functional state switching of cadherins.Dev Dyn. 2002 May;224(1):18-29. doi: 10.1002/dvdy.10087. Dev Dyn. 2002. PMID: 11984870 Review.
-
Roles for the protein tyrosine phosphatase SHP-2 in cytoskeletal organization, cell adhesion and cell migration revealed by overexpression of a dominant negative mutant.Oncogene. 2000 Jan 6;19(1):75-84. doi: 10.1038/sj.onc.1203204. Oncogene. 2000. PMID: 10644982
-
Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways.Trends Cell Biol. 2006 Apr;16(4):213-23. doi: 10.1016/j.tcb.2006.02.005. Epub 2006 Mar 10. Trends Cell Biol. 2006. PMID: 16529933 Review.
Cited by
-
Effects of NRP1 on angiogenesis and vascular maturity in endothelial cells are dependent on the expression of SEMA4D.Int J Mol Med. 2020 Oct;46(4):1321-1334. doi: 10.3892/ijmm.2020.4692. Epub 2020 Aug 3. Int J Mol Med. 2020. PMID: 32945351 Free PMC article.
-
Group II p21-activated kinase, PAK4, is needed for activation of focal adhesion kinases, MAPK, GSK3, and β-catenin in rat pancreatic acinar cells.Am J Physiol Gastrointest Liver Physiol. 2020 Mar 1;318(3):G490-G503. doi: 10.1152/ajpgi.00229.2019. Epub 2020 Jan 27. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 31984786 Free PMC article.
-
Occludin phosphorylation in regulation of epithelial tight junctions.Ann N Y Acad Sci. 2009 May;1165:62-8. doi: 10.1111/j.1749-6632.2009.04054.x. Ann N Y Acad Sci. 2009. PMID: 19538289 Free PMC article.
-
Immunopathogenesis and therapeutic approaches in pediatric celiac disease.Expert Rev Clin Immunol. 2016 Aug;12(8):857-69. doi: 10.1586/1744666X.2016.1168294. Epub 2016 Apr 1. Expert Rev Clin Immunol. 2016. PMID: 26999328 Free PMC article. Review.
-
R-PTP-κ Inhibits Contact-Dependent Cell Growth by Suppressing E2F Activity.Biomedicines. 2022 Dec 9;10(12):3199. doi: 10.3390/biomedicines10123199. Biomedicines. 2022. PMID: 36551956 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
