ConSurf 2005: the projection of evolutionary conservation scores of residues on protein structures
- PMID: 15980475
- PMCID: PMC1160131
- DOI: 10.1093/nar/gki370
ConSurf 2005: the projection of evolutionary conservation scores of residues on protein structures
Abstract
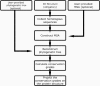
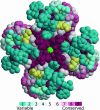
Key amino acid positions that are important for maintaining the 3D structure of a protein and/or its function(s), e.g. catalytic activity, binding to ligand, DNA or other proteins, are often under strong evolutionary constraints. Thus, the biological importance of a residue often correlates with its level of evolutionary conservation within the protein family. ConSurf (http://consurf.tau.ac.il/) is a web-based tool that automatically calculates evolutionary conservation scores and maps them on protein structures via a user-friendly interface. Structurally and functionally important regions in the protein typically appear as patches of evolutionarily conserved residues that are spatially close to each other. We present here version 3.0 of ConSurf. This new version includes an empirical Bayesian method for scoring conservation, which is more accurate than the maximum-likelihood method that was used in the earlier release. Various additional steps in the calculation can now be controlled by a number of advanced options, thus further improving the accuracy of the calculation. Moreover, ConSurf version 3.0 also includes a measure of confidence for the inferred amino acid conservation scores.
Figures
Similar articles
-
The ConSurf-HSSP database: the mapping of evolutionary conservation among homologs onto PDB structures.Proteins. 2005 Feb 15;58(3):610-7. doi: 10.1002/prot.20305. Proteins. 2005. PMID: 15614759
-
ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids.Nucleic Acids Res. 2010 Jul;38(Web Server issue):W529-33. doi: 10.1093/nar/gkq399. Epub 2010 May 16. Nucleic Acids Res. 2010. PMID: 20478830 Free PMC article.
-
ConSurf: identification of functional regions in proteins by surface-mapping of phylogenetic information.Bioinformatics. 2003 Jan;19(1):163-4. doi: 10.1093/bioinformatics/19.1.163. Bioinformatics. 2003. PMID: 12499312
-
Scoring residue conservation.Proteins. 2002 Aug 1;48(2):227-41. doi: 10.1002/prot.10146. Proteins. 2002. PMID: 12112692 Review.
-
Wenxiang 3.0: Evolutionary Visualization of α, π, and 3/10 Helices.Evol Bioinform Online. 2022 May 31;18:11769343221101014. doi: 10.1177/11769343221101014. eCollection 2022. Evol Bioinform Online. 2022. PMID: 35668741 Free PMC article. Review.
Cited by
-
Neuropilins lock secreted semaphorins onto plexins in a ternary signaling complex.Nat Struct Mol Biol. 2012 Dec;19(12):1293-9. doi: 10.1038/nsmb.2416. Epub 2012 Oct 28. Nat Struct Mol Biol. 2012. PMID: 23104057 Free PMC article.
-
Analysis of conformational motions and residue fluctuations for Escherichia coli ribose-binding protein revealed with elastic network models.Int J Mol Sci. 2013 May 21;14(5):10552-69. doi: 10.3390/ijms140510552. Int J Mol Sci. 2013. PMID: 23698778 Free PMC article.
-
Coronavirus-Specific Antibody Cross Reactivity in Rhesus Macaques Following SARS-CoV-2 Vaccination and Infection.J Virol. 2021 May 10;95(11):e00117-21. doi: 10.1128/JVI.00117-21. Epub 2021 Mar 10. J Virol. 2021. PMID: 33692201 Free PMC article.
-
PI2PE: protein interface/interior prediction engine.Nucleic Acids Res. 2007 Jul;35(Web Server issue):W357-62. doi: 10.1093/nar/gkm231. Epub 2007 May 25. Nucleic Acids Res. 2007. PMID: 17526530 Free PMC article.
-
Homozygous GRHPR C.494G>A mutation is deleterious that causes early onset of nephrolithiasis in West Bengal, India.Front Mol Biosci. 2022 Dec 22;9:1049620. doi: 10.3389/fmolb.2022.1049620. eCollection 2022. Front Mol Biosci. 2022. PMID: 36619171 Free PMC article.
References
-
- Glaser F., Pupko T., Paz I., Bell R.E., Bechor-Shental D., Martz E., Ben-Tal N. ConSurf: identification of functional regions in proteins by surface-mapping of phylogenetic information. Bioinformatics. 2003;19:163–164. - PubMed
-
- Mayrose I., Graur D., Ben-Tal N., Pupko T. Comparison of site-specific rate-inference methods for protein sequences: empirical Bayesian methods are superior. Mol. Biol. Evol. 2004;21:1781–1791. - PubMed
-
- Pupko T., Bell R.E., Mayrose I., Glaser F., Ben-Tal N. Rate4Site: an algorithmic tool for the identification of functional regions in proteins by surface mapping of evolutionary determinants within their homologues. Bioinformatics. 2002;18:S71–S77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

