Abl tyrosine kinase and its substrate Ena/VASP have functional interactions with kinesin-1
- PMID: 15975902
- PMCID: PMC1196332
- DOI: 10.1091/mbc.e05-02-0116
Abl tyrosine kinase and its substrate Ena/VASP have functional interactions with kinesin-1
Abstract
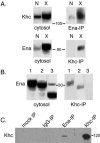
Relatively little is known about how microtubule motors are controlled or about how the functions of different cytoskeletal systems are integrated. A yeast two-hybrid screen for proteins that bind to Drosophila Enabled (Ena), an actin polymerization factor that is negatively regulated by Abl tyrosine kinase, identified kinesin heavy chain (Khc), a member of the kinesin-1 subfamily of microtubule motors. Coimmunoprecipitation from Drosophila cytosol confirmed a physical interaction between Khc and Ena. Kinesin-1 motors can carry organelles and other macromolecular cargoes from neuronal cell bodies toward terminals in fast-axonal-transport. Ena distribution in larval axons was not affected by mutations in the Khc gene, suggesting that Ena is not itself a fast transport cargo of Drosophila kinesin-1. Genetic interaction tests showed that in a background sensitized by reduced Khc gene dosage, a reduction in Abl gene dosage caused distal paralysis and axonal swellings. A concomitant reduction in ena dosage rescued those defects. These results suggest that Ena/VASP, when not inhibited by the Abl pathway, can bind Khc and reduce its transport activity in axons.
Figures




Similar articles
-
Abl-1-bridged tyrosine phosphorylation of VASP by Abelson kinase impairs association of VASP to focal adhesions and regulates leukaemic cell adhesion.Biochem J. 2012 Feb 1;441(3):889-99. doi: 10.1042/BJ20110951. Biochem J. 2012. PMID: 22014333
-
Abi plays an opposing role to Abl in Drosophila axonogenesis and synaptogenesis.Development. 2009 Sep;136(18):3099-107. doi: 10.1242/dev.033324. Epub 2009 Aug 12. Development. 2009. PMID: 19675132
-
Mutations in Drosophila enabled and rescue by human vasodilator-stimulated phosphoprotein (VASP) indicate important functional roles for Ena/VASP homology domain 1 (EVH1) and EVH2 domains.Mol Biol Cell. 1998 Aug;9(8):2157-71. doi: 10.1091/mbc.9.8.2157. Mol Biol Cell. 1998. PMID: 9693373 Free PMC article.
-
Actin-based motility: stop and go with Ena/VASP proteins.Trends Biochem Sci. 2001 Apr;26(4):243-9. doi: 10.1016/s0968-0004(00)01785-0. Trends Biochem Sci. 2001. PMID: 11295557 Review.
-
From Abl to actin: Abl tyrosine kinase and associated proteins in growth cone motility.Curr Opin Neurobiol. 2000 Feb;10(1):80-7. doi: 10.1016/s0959-4388(99)00058-6. Curr Opin Neurobiol. 2000. PMID: 10679439 Review.
Cited by
-
Regulation of adherens junction dynamics by phosphorylation switches.J Signal Transduct. 2012;2012:125295. doi: 10.1155/2012/125295. Epub 2012 Jul 12. J Signal Transduct. 2012. PMID: 22848810 Free PMC article.
-
miR-8 controls synapse structure by repression of the actin regulator enabled.Development. 2014 May;141(9):1864-74. doi: 10.1242/dev.105791. Epub 2014 Apr 9. Development. 2014. PMID: 24718988 Free PMC article.
-
A functional misexpression screen uncovers a role for enabled in progressive neurodegeneration.PLoS One. 2008 Oct 8;3(10):e3332. doi: 10.1371/journal.pone.0003332. PLoS One. 2008. PMID: 18841196 Free PMC article.
-
The axonal transport of mitochondria.J Cell Sci. 2005 Dec 1;118(Pt 23):5411-9. doi: 10.1242/jcs.02745. J Cell Sci. 2005. PMID: 16306220 Free PMC article. Review.
-
APLIP1, a kinesin binding JIP-1/JNK scaffold protein, influences the axonal transport of both vesicles and mitochondria in Drosophila.Curr Biol. 2005 Dec 6;15(23):2137-41. doi: 10.1016/j.cub.2005.10.047. Curr Biol. 2005. PMID: 16332540 Free PMC article.
References
-
- Ahern-Djamali, S. M., Comer, A. R., Bachmann, C., Kastenmeier, A. S., Reddy, S. K., Beckerle, M. C., Walter, U., and Hoffmann, F. M. (1998). Mutations in Drosophila enabled and rescue by human vasodilator-stimulated phosphoprotein (VASP) indicate important functional roles for Ena/VASP homology domain 1 (EVH1) and EVH2 domains. Mol. Biol. Cell 9, 2157-2171. - PMC - PubMed
-
- Bear, J. E. et al. (2002). Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell 109, 509-521. - PubMed
-
- Bowman, A. B., Patel-King, R. S., Benashski, S. E., McCaffery, J. M., Goldstein, L.S.B., and King, S. M. (1999). Drosophila roadblock and Chlamydomonas LC7, a conserved family of dynein-associated proteins involved in axonal transport, flagellar motility, and mitosis. J. Cell Biol. 146, 165-179. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

