A Rab21/LIM-only/CH-LIM complex regulates phagocytosis via both activating and inhibitory mechanisms
- PMID: 15962002
- PMCID: PMC1173156
- DOI: 10.1038/sj.emboj.7600716
A Rab21/LIM-only/CH-LIM complex regulates phagocytosis via both activating and inhibitory mechanisms
Abstract
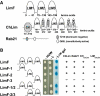
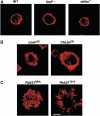
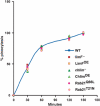
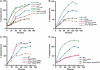
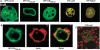
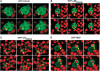
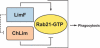
We have identified two LIM domain proteins, LimF and ChLim, from Dictyostelium that interact with each other and with the small, Rab5-related, Rab21 GTPase to collectively regulate phagocytosis. To investigate in vivo functions, we generated cell lines that lack or overexpress LimF and ChLim and strains that express activating or inhibiting variants of Rab21. Overexpression of LimF, loss of ChLim, or expression of constitutively active Rab21 increases the rate of phagocytosis above that of wild type. Conversely, loss of LimF, overexpression of ChLim, or expression of a dominant-negative Rab21 inhibits phagocytosis. Our studies using cells carrying multiple mutations in these genes further indicate that ChLim antagonizes the activating function of Rab21-GTP during phagocytosis; in turn, LimF is required for Rab21-GTP function. Finally, we demonstrate that ChLim and LimF localize to the phagocytic cup and phago-lysosomal vesicles. We suggest that LimF, ChLim, and activated Rab21-GTP participate as a novel signaling complex that regulates phagocytic activity.
Figures

Similar articles
-
Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins.J Cell Biol. 2006 Jun 5;173(5):767-80. doi: 10.1083/jcb.200509019. J Cell Biol. 2006. PMID: 16754960 Free PMC article.
-
A role for the small GTPase Rab21 in the early endocytic pathway.J Cell Sci. 2004 Dec 15;117(Pt 26):6297-311. doi: 10.1242/jcs.01560. Epub 2004 Nov 23. J Cell Sci. 2004. PMID: 15561770
-
Varp is a Rab21 guanine nucleotide exchange factor and regulates endosome dynamics.J Cell Sci. 2006 Mar 15;119(Pt 6):1053-62. doi: 10.1242/jcs.02810. J Cell Sci. 2006. PMID: 16525121
-
Cell-cell signaling and adhesion in phagocytosis and early development of Dictyostelium.Int J Dev Biol. 2000;44(6):733-42. Int J Dev Biol. 2000. PMID: 11061438 Review.
-
The Regulatory Mechanism of Rab21 in Human Diseases.Mol Neurobiol. 2023 Oct;60(10):5944-5953. doi: 10.1007/s12035-023-03454-0. Epub 2023 Jun 28. Mol Neurobiol. 2023. PMID: 37369821 Review.
Cited by
-
The balance in the delivery of ER components and the vacuolar proton pump to the phagosome depends on myosin IK in Dictyostelium.Mol Cell Proteomics. 2012 Oct;11(10):886-900. doi: 10.1074/mcp.M112.017608. Epub 2012 Jun 26. Mol Cell Proteomics. 2012. PMID: 22736568 Free PMC article.
-
Dynamic changes in the spatiotemporal localization of Rab21 in live RAW264 cells during macropinocytosis.PLoS One. 2009 Aug 20;4(8):e6689. doi: 10.1371/journal.pone.0006689. PLoS One. 2009. PMID: 19693279 Free PMC article.
-
Rab GTPases take centre stage in understanding Entamoeba histolytica biology.Small GTPases. 2020 Sep;11(5):320-333. doi: 10.1080/21541248.2018.1528840. Epub 2018 Oct 13. Small GTPases. 2020. PMID: 30273093 Free PMC article. Review.
-
Phagocytosis by the Retinal Pigment Epithelium: Recognition, Resolution, Recycling.Front Immunol. 2020 Nov 13;11:604205. doi: 10.3389/fimmu.2020.604205. eCollection 2020. Front Immunol. 2020. PMID: 33281830 Free PMC article. Review.
-
TOR complex 2 (TORC2) in Dictyostelium suppresses phagocytic nutrient capture independently of TORC1-mediated nutrient sensing.J Cell Sci. 2012 Jan 1;125(Pt 1):37-48. doi: 10.1242/jcs.077040. Epub 2012 Jan 20. J Cell Sci. 2012. PMID: 22266904 Free PMC article.
References
-
- Bush J, Franek K, Daniel J, Spiegelman GB, Weeks G, Cardelli J (1993) Cloning and characterization of five novel Dictyostelium discoideum rab-related genes. Gene 136: 55–60 - PubMed
-
- Cardelli J (2001) Phagocytosis and macropinocytosis in Dictyostelium: phosphoinositide-based processes, biochemically distinct. Traffic 2: 311–320 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

