Analysis of foot-and-mouth disease virus internalization events in cultured cells
- PMID: 15956593
- PMCID: PMC1143741
- DOI: 10.1128/JVI.79.13.8506-8518.2005
Analysis of foot-and-mouth disease virus internalization events in cultured cells
Abstract
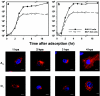
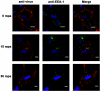
It has been demonstrated that foot-and-mouth disease virus (FMDV) can utilize at least four members of the alpha(V) subgroup of the integrin family of receptors in vitro. The virus interacts with these receptors via a highly conserved arginine-glycine-aspartic acid amino acid sequence motif located within the betaG-betaH loop of VP1. While there have been extensive studies of virus-receptor interactions at the cell surface, our understanding of the events during viral entry into the infected cell is still not clear. We have utilized confocal microscopy to analyze the entry of two FMDV serotypes (types A and O) after interaction with integrin receptors at the cell surface. In cell cultures expressing both the alphaVbeta3 and alphaVbeta6 integrins, virus adsorbed to the cells at 4 degrees C appears to colocalize almost exclusively with the alphaVbeta6 integrin. Upon shifting the infected cells to 37 degrees C, FMDV capsid proteins were detected within 15 min after the temperature shift, in association with the integrin in vesicular structures that were positive for a marker of clathrin-mediated endocytosis. In contrast, virus did not colocalize with a marker for caveola-mediated endocytosis. Virus remained associated with the integrin until about 1 h after the temperature shift, when viral proteins appeared around the perinuclear region of the cell. By 15 min after the temperature shift, viral proteins were seen colocalizing with a marker for early endosomes, while no colocalization with late endosomal markers was observed. In the presence of monensin, which raises the pH of endocytic vesicles and has been shown to inhibit FMDV replication, viral proteins were not released from the recycling endosome structures. Viral proteins were not observed associated with the endoplasmic reticulum or the Golgi. These data indicate that FMDV utilizes the clathrin-mediated endocytosis pathway to infect the cells and that viral replication begins due to acidification of endocytic vesicles, causing the breakdown of the viral capsid structure and release of the genome by an as-yet-unidentified mechanism.
Figures

Similar articles
-
Analysis of foot-and-mouth disease virus integrin receptor expression in tissues from naïve and infected cattle.J Comp Pathol. 2009 Aug-Oct;141(2-3):98-112. doi: 10.1016/j.jcpa.2008.09.008. Epub 2009 Jun 9. J Comp Pathol. 2009. PMID: 19515380
-
Interactions of foot-and-mouth disease virus with soluble bovine alphaVbeta3 and alphaVbeta6 integrins.J Virol. 2004 Sep;78(18):9773-81. doi: 10.1128/JVI.78.18.9773-9781.2004. J Virol. 2004. PMID: 15331710 Free PMC article.
-
Cellular receptors for foot and mouth disease virus.Intervirology. 2009;52(4):201-12. doi: 10.1159/000226121. Epub 2009 Jun 24. Intervirology. 2009. PMID: 19556802 Review.
-
A second RGD motif in the 1D capsid protein of a SAT1 type foot-and-mouth disease virus field isolate is not essential for attachment to target cells.Virus Res. 2007 Mar;124(1-2):184-92. doi: 10.1016/j.virusres.2006.11.003. Epub 2006 Dec 11. Virus Res. 2007. PMID: 17161881
-
Action of mutagenic agents and antiviral inhibitors on foot-and-mouth disease virus.Virus Res. 2005 Feb;107(2):183-93. doi: 10.1016/j.virusres.2004.11.008. Virus Res. 2005. PMID: 15649564 Review.
Cited by
-
Entry of feline calicivirus is dependent on clathrin-mediated endocytosis and acidification in endosomes.J Virol. 2006 Aug;80(15):7500-9. doi: 10.1128/JVI.02452-05. J Virol. 2006. PMID: 16840330 Free PMC article.
-
Specificity of the VP1 GH loop of Foot-and-Mouth Disease virus for alphav integrins.J Virol. 2006 Oct;80(19):9798-810. doi: 10.1128/JVI.00577-06. J Virol. 2006. PMID: 16973584 Free PMC article.
-
Foot-and-mouth disease virus utilizes an autophagic pathway during viral replication.Virology. 2011 Feb 5;410(1):142-50. doi: 10.1016/j.virol.2010.10.042. Epub 2010 Nov 26. Virology. 2011. PMID: 21112602 Free PMC article.
-
VP2 mediates the release of the feline calicivirus RNA genome by puncturing the endosome membrane of infected cells.J Virol. 2024 May 14;98(5):e0035024. doi: 10.1128/jvi.00350-24. Epub 2024 Apr 9. J Virol. 2024. PMID: 38591900 Free PMC article.
-
A dominant-negative mutant of rab5 inhibits infection of cells by foot-and-mouth disease virus: implications for virus entry.J Virol. 2009 Jun;83(12):6247-56. doi: 10.1128/JVI.02460-08. Epub 2009 Apr 8. J Virol. 2009. PMID: 19357169 Free PMC article.
References
-
- Basu, S. K., J. L. Goldstein, R. G. Anderson, and M. S. Brown. 1981. Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell 24:493-502. - PubMed
-
- Baxt, B. 1987. Effect of lysosomotropic compounds on early events in foot-and-mouth disease virus replication. Virus Res. 7:257-271. - PubMed
-
- Baxt, B., and H. L. Bachrach. 1982. The adsorption and degradation of foot-and-mouth disease virus by isolated BHK-21 cell plasma membranes. Virology 116:391-405. - PubMed
-
- Baxt, B., and H. L. Bachrach. 1980. Early interactions of foot-and-mouth disease virus with cultured cells. Virology 104:42-55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

