Chemokine-induced recruitment of genetically modified bone marrow cells into the CNS of GM1-gangliosidosis mice corrects neuronal pathology
- PMID: 15941905
- PMCID: PMC1895262
- DOI: 10.1182/blood-2005-03-1189
Chemokine-induced recruitment of genetically modified bone marrow cells into the CNS of GM1-gangliosidosis mice corrects neuronal pathology
Abstract
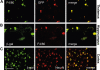
Bone marrow cells (BMCs) could correct some pathologic conditions of the central nervous system (CNS) if these cells would effectively repopulate the brain. One such condition is G(M1)-gangliosidosis, a neurodegenerative glycosphingolipidosis due to deficiency of lysosomal beta-galactosidase (beta-gal). In this disease, abnormal build up of G(M1)-ganglioside in the endoplasmic reticulum of brain cells results in calcium imbalance, induction of an unfolded protein response (UPR), and neuronal apoptosis. These processes are accompanied by the activation/proliferation of microglia and the production of inflammatory cytokines. Here we demonstrate that local neuroinflammation promotes the selective activation of chemokines, such as stromal-cell-derived factor 1 (SDF-1), macrophage inflammatory protein 1-alpha (MIP-1alpha), and MIP-1beta, which chemoattract genetically modified BMCs into the CNS. Mice that underwent bone marrow transplantation showed increased beta-gal activity in different brain regions and reduced lysosomal storage. Decreased production of chemokines and effectors of the UPR as well as restoration of neurologic functions accompanied this phenotypic reversion. Our results suggest that beta-gal-expressing bone marrow (BM)-derived cells selectively migrate to the CNS under a gradient of chemokines and become a source of correcting enzyme to deficient neurons. Thus, a disease condition such as G(M1)-gangliosidosis, which is characterized by neurodegeneration and neuroinflammation, may influence the response of the CNS to ex vivo gene therapy.
Figures
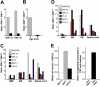
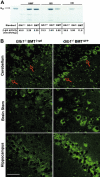
 ) and 4 months (▪) of age. SDF-1α and SDF-1β mRNAs were higher in Glb1-/- mice than in Glb1+/+ mice. The reactions were standardized to the level of GAPDH mRNA. (B) ELISA of SDF-1α protein in Glb1-/- cerebellum extracts showed that relatively more SDF-1α was expressed at 3 months (
) and 4 months (▪) of age. SDF-1α and SDF-1β mRNAs were higher in Glb1-/- mice than in Glb1+/+ mice. The reactions were standardized to the level of GAPDH mRNA. (B) ELISA of SDF-1α protein in Glb1-/- cerebellum extracts showed that relatively more SDF-1α was expressed at 3 months ( ) than at 4 months (▪) of age. (C-D) RNase protection assay revealed that the β-chemokines RANTES, MIP-1β, MIP-1α, IP-10, MCP-1, and TCA-3 were up-regulated in hindbrain, midbrain, and forebrain regions (HMF); brain stem (BS); cerebellum (CB); and spinal cord of 3- (C) and 4-month-old (D) Glb1-/- mice compared with those of Glb1+/+ controls. The relative levels of RNA induction were normalized against L32 RNA. The amount of chemokines expressed is reported as fold increase over that detected in age-matched Glb1+/+ mice. (E) CSF from 3-month-old Glb1-/- mice contained more white blood cells (WBCs) than did the CSF from PPCA-/- mice or Glb1+/+ littermates. (F) Transmigration assay demonstrated increased monocyte migration toward Glb1-/- CSF than toward Glb1+/+ CSF. Nonspecific cell migration toward serum-free medium was used as a negative control. Data are expressed as mean ± standard deviation (SD).
) than at 4 months (▪) of age. (C-D) RNase protection assay revealed that the β-chemokines RANTES, MIP-1β, MIP-1α, IP-10, MCP-1, and TCA-3 were up-regulated in hindbrain, midbrain, and forebrain regions (HMF); brain stem (BS); cerebellum (CB); and spinal cord of 3- (C) and 4-month-old (D) Glb1-/- mice compared with those of Glb1+/+ controls. The relative levels of RNA induction were normalized against L32 RNA. The amount of chemokines expressed is reported as fold increase over that detected in age-matched Glb1+/+ mice. (E) CSF from 3-month-old Glb1-/- mice contained more white blood cells (WBCs) than did the CSF from PPCA-/- mice or Glb1+/+ littermates. (F) Transmigration assay demonstrated increased monocyte migration toward Glb1-/- CSF than toward Glb1+/+ CSF. Nonspecific cell migration toward serum-free medium was used as a negative control. Data are expressed as mean ± standard deviation (SD).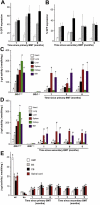
 ), and WBCs (▪) demonstrated consistent, long-term expression of the transgene as long as 6 months after primary transplantation (1 month, n = 7; 3 months, n = 10; 6 months, n = 6) and as long as 9 months after secondary transplantation (1 month, n = 3; 3 months, n = 3; 6 months, n = 3; 9 months, n = 3). (C-D) Analyses of β-gal activity in systemic tissues of treated mice revealed a significantly higher level of expression of the corrective enzyme after primary (Glb1+/+, n = 7; Glb1-/-, n = 5; 1 month, n = 7; 3 months, n = 8; 6 months, n = 8) and secondary transplantation (Glb1+/+, n = 7; Glb1-/-, n = 3; 1 month, n = 3; 3 months, n = 3; 6 months, n = 3; 9 months, n = 3) compared with Glb1-/- untreated mice. (E) The β-gal activity was higher in the HMF (hindbrain, midbrain, and forebrain), brain stem, cerebellum, and spinal cord of Glb1-/- mice that underwent primary transplantation and in the brainstem, cerebellum, and spinal cord of Glb1-/- mice that underwent secondary transplantation compared with untreated Glb1-/- mice. Data are expressed as mean ± SD; groups were compared by one-way repeated measures analysis of variance (ANOVA). *P < .001 and #P < .05 relative to untreated Glb1-/- littermates at the same age; post hoc Tukey test.
), and WBCs (▪) demonstrated consistent, long-term expression of the transgene as long as 6 months after primary transplantation (1 month, n = 7; 3 months, n = 10; 6 months, n = 6) and as long as 9 months after secondary transplantation (1 month, n = 3; 3 months, n = 3; 6 months, n = 3; 9 months, n = 3). (C-D) Analyses of β-gal activity in systemic tissues of treated mice revealed a significantly higher level of expression of the corrective enzyme after primary (Glb1+/+, n = 7; Glb1-/-, n = 5; 1 month, n = 7; 3 months, n = 8; 6 months, n = 8) and secondary transplantation (Glb1+/+, n = 7; Glb1-/-, n = 3; 1 month, n = 3; 3 months, n = 3; 6 months, n = 3; 9 months, n = 3) compared with Glb1-/- untreated mice. (E) The β-gal activity was higher in the HMF (hindbrain, midbrain, and forebrain), brain stem, cerebellum, and spinal cord of Glb1-/- mice that underwent primary transplantation and in the brainstem, cerebellum, and spinal cord of Glb1-/- mice that underwent secondary transplantation compared with untreated Glb1-/- mice. Data are expressed as mean ± SD; groups were compared by one-way repeated measures analysis of variance (ANOVA). *P < .001 and #P < .05 relative to untreated Glb1-/- littermates at the same age; post hoc Tukey test.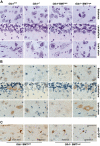
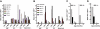
 ; SDF-1β, □) were higher than those in treated Glb1-/- mice (SDF-1α, ▪; SDF-1β,
; SDF-1β, □) were higher than those in treated Glb1-/- mice (SDF-1α, ▪; SDF-1β,  ). (D) The amount of SDF-1α protein in the cerebellum was also lower in treated Glb1-/- mice (▪) than it was in untreated Glb1/- mice (
). (D) The amount of SDF-1α protein in the cerebellum was also lower in treated Glb1-/- mice (▪) than it was in untreated Glb1/- mice ( ). Data are expressed as mean ± SD.
). Data are expressed as mean ± SD.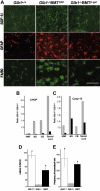
 ). (D) Glb1-/- mice that underwent transplantation (▪) performed better than untreated Glb1-/- mice (
). (D) Glb1-/- mice that underwent transplantation (▪) performed better than untreated Glb1-/- mice ( ) but not as well as wild-type mice (□) on the rotating rod test of motor coordination and balance (n = 15). (E) Similar results were seen on the open-field exploratory activity test (n = 17). Data are expressed as mean ± SD; groups were compared by one-way repeated measures ANOVA. #P < .05 relative to untreated Glb1-/- littermates at the same age; post hoc Tukey test.
) but not as well as wild-type mice (□) on the rotating rod test of motor coordination and balance (n = 15). (E) Similar results were seen on the open-field exploratory activity test (n = 17). Data are expressed as mean ± SD; groups were compared by one-way repeated measures ANOVA. #P < .05 relative to untreated Glb1-/- littermates at the same age; post hoc Tukey test.Similar articles
-
CCR1 chemokines promote the chemotactic recruitment, RANKL development, and motility of osteoclasts and are induced by inflammatory cytokines in osteoblasts.J Bone Miner Res. 2004 Dec;19(12):2065-77. doi: 10.1359/JBMR.040910. Epub 2004 Sep 20. J Bone Miner Res. 2004. PMID: 15537451
-
GM1-ganglioside-mediated activation of the unfolded protein response causes neuronal death in a neurodegenerative gangliosidosis.Mol Cell. 2004 Sep 10;15(5):753-66. doi: 10.1016/j.molcel.2004.08.029. Mol Cell. 2004. PMID: 15350219
-
Preclinical Enzyme Replacement Therapy with a Recombinant β-Galactosidase-Lectin Fusion for CNS Delivery and Treatment of GM1-Gangliosidosis.Cells. 2022 Aug 19;11(16):2579. doi: 10.3390/cells11162579. Cells. 2022. PMID: 36010656 Free PMC article.
-
Relationship between the chemokine receptor CCR5 and microglia in neurological disorders: consequences of targeting CCR5 on neuroinflammation, neuronal death and regeneration in a model of epilepsy.CNS Neurol Disord Drug Targets. 2013 Sep;12(6):815-29. doi: 10.2174/18715273113126660173. CNS Neurol Disord Drug Targets. 2013. PMID: 24047524 Review.
-
Neuronal chemokines: versatile messengers in central nervous system cell interaction.Mol Neurobiol. 2007 Oct;36(2):137-51. doi: 10.1007/s12035-007-0036-8. Epub 2007 Jul 10. Mol Neurobiol. 2007. PMID: 17952658 Free PMC article. Review.
Cited by
-
Stem cells and neurological diseases.Cell Prolif. 2008 Feb;41 Suppl 1(Suppl 1):94-114. doi: 10.1111/j.1365-2184.2008.00486.x. Cell Prolif. 2008. PMID: 18181951 Free PMC article. Review.
-
Abnormal autophagy, ubiquitination, inflammation and apoptosis are dependent upon lysosomal storage and are useful biomarkers of mucopolysaccharidosis VI.Pathogenetics. 2009 Jun 16;2(1):4. doi: 10.1186/1755-8417-2-4. Pathogenetics. 2009. PMID: 19531206 Free PMC article.
-
Axonopathy and Reduction of Membrane Resistance: Key Features in a New Murine Model of Human GM1-Gangliosidosis.J Clin Med. 2020 Apr 2;9(4):1004. doi: 10.3390/jcm9041004. J Clin Med. 2020. PMID: 32252429 Free PMC article.
-
Activation of CXCL10/CXCR3 signaling attenuates morphine analgesia: involvement of Gi protein.J Mol Neurosci. 2014 Aug;53(4):571-9. doi: 10.1007/s12031-013-0223-1. Epub 2014 Jan 12. J Mol Neurosci. 2014. PMID: 24415274
-
Therapeutic developments for neurodegenerative GM1 gangliosidosis.Front Neurosci. 2024 Apr 26;18:1392683. doi: 10.3389/fnins.2024.1392683. eCollection 2024. Front Neurosci. 2024. PMID: 38737101 Free PMC article. Review.
References
-
- Santamaria P. Cytokines and chemokines in autoimmune disease: an overview. Adv Exp Med Biol. 2003;520: 1-7. - PubMed
-
- Stoll G, Jander S, Schroeter M. Detrimental and beneficial effects of injury-induced inflammation and cytokine expression in the nervous system. Adv Exp Med Biol. 2002;513: 87-113. - PubMed
-
- Mennicken F, Maki R, de Souza EB, Quirion R. Chemokines and chemokine receptors in the CNS: a possible role in neuroinflammation and patterning. Trends Pharmacol Sci. 1999;20: 73-78. - PubMed
-
- Kremlev SG, Roberts RL, Palmer C. Differential expression of chemokines and chemokine receptors during microglial activation and inhibition. J Neuroimmunol. 2004;149: 1-9. - PubMed
-
- Gourmala NG, Limonta S, Bochelen D, Sauter A, Boddeke HW. Localization of macrophage inflammatory protein: macrophage inflammatory protein-1 expression in rat brain after peripheral administration of lipopolysaccharide and focal cerebral ischemia. Neuroscience. 1999;88: 1255-1266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

