Interaction between the human cytomegalovirus UL82 gene product (pp71) and hDaxx regulates immediate-early gene expression and viral replication
- PMID: 15919932
- PMCID: PMC1143679
- DOI: 10.1128/JVI.79.12.7792-7802.2005
Interaction between the human cytomegalovirus UL82 gene product (pp71) and hDaxx regulates immediate-early gene expression and viral replication
Abstract
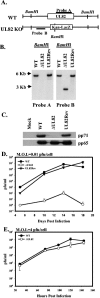
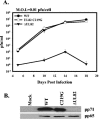
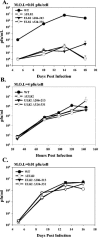
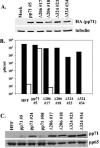
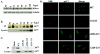
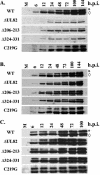
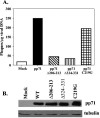
The human cytomegalovirus UL82-encoded pp71 protein is required for efficient virus replication and immediate-early gene expression when cells are infected at a low multiplicity. Functions attributed to pp71 include the ability to enhance the infectivity of viral DNA, bind to and target hypophosphorylated Rb family member proteins for degradation, drive quiescent cells into the cell cycle, and bind to the cellular protein hDaxx. Using UL82 mutant viruses, we demonstrate that the LXCXD motif within pp71 is not necessary for efficient virus replication in fibroblasts, suggesting that pp71's ability to degrade hypophosphorylated Rb family members and induce quiescent cells into the cell cycle is not responsible for the growth defect associated with a UL82 deletion mutant. However, UL82 mutants that cannot bind to hDaxx are unable to induce immediate-early gene expression and are severely attenuated for viral replication. These results indicate that the interaction between the human cytomegalovirus UL82 gene product (pp71) and hDaxx regulates immediate-early gene expression and viral replication.
Figures

Similar articles
-
Human cytomegalovirus (HCMV) UL82 gene product (pp71) relieves hDaxx-mediated repression of HCMV replication.J Virol. 2006 Jun;80(12):6188-91. doi: 10.1128/JVI.02676-05. J Virol. 2006. PMID: 16731959 Free PMC article.
-
Role of the cellular protein hDaxx in human cytomegalovirus immediate-early gene expression.J Gen Virol. 2006 May;87(Pt 5):1113-1121. doi: 10.1099/vir.0.81566-0. J Gen Virol. 2006. PMID: 16603511
-
Human cytomegalovirus protein pp71 displaces the chromatin-associated factor ATRX from nuclear domain 10 at early stages of infection.J Virol. 2008 Dec;82(24):12543-54. doi: 10.1128/JVI.01215-08. Epub 2008 Oct 15. J Virol. 2008. PMID: 18922870 Free PMC article.
-
Intrinsic cellular defense mechanisms targeting human cytomegalovirus.Virus Res. 2011 May;157(2):128-33. doi: 10.1016/j.virusres.2010.10.002. Epub 2010 Oct 8. Virus Res. 2011. PMID: 20934469 Review.
-
Expanding the Known Functional Repertoire of the Human Cytomegalovirus pp71 Protein.Front Cell Infect Microbiol. 2020 Mar 12;10:95. doi: 10.3389/fcimb.2020.00095. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32226778 Free PMC article. Review.
Cited by
-
Human cytomegalovirus persistence.Cell Microbiol. 2012 May;14(5):644-55. doi: 10.1111/j.1462-5822.2012.01774.x. Epub 2012 Mar 8. Cell Microbiol. 2012. PMID: 22329758 Free PMC article. Review.
-
Chromatin-Remodeling Factor SPOC1 Acts as a Cellular Restriction Factor against Human Cytomegalovirus by Repressing the Major Immediate Early Promoter.J Virol. 2018 Jun 29;92(14):e00342-18. doi: 10.1128/JVI.00342-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29743358 Free PMC article.
-
Ubiquitin-independent proteasomal degradation during oncogenic viral infections.Biochim Biophys Acta. 2011 Dec;1816(2):147-57. doi: 10.1016/j.bbcan.2011.05.005. Epub 2011 Jun 6. Biochim Biophys Acta. 2011. PMID: 21664948 Free PMC article. Review.
-
Tuning the Orchestra: HCMV vs. Innate Immunity.Front Microbiol. 2020 Apr 15;11:661. doi: 10.3389/fmicb.2020.00661. eCollection 2020. Front Microbiol. 2020. PMID: 32351486 Free PMC article. Review.
-
Emerging Mechanisms of G1/S Cell Cycle Control by Human and Mouse Cytomegaloviruses.mBio. 2021 Dec 21;12(6):e0293421. doi: 10.1128/mBio.02934-21. Epub 2021 Dec 14. mBio. 2021. PMID: 34903047 Free PMC article. Review.
References
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
-
- Bresnahan, W. A., I. Boldogh, T. Ma, T. Albrecht, and E. A. Thompson. 1996. Cyclin E/Cdk2 activity is controlled by different mechanisms in the G0 and G1 phases of the cell cycle. Cell Growth Differ. 7:1283-1290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

