The molecular pharmacology and cell biology of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors
- PMID: 15914469
- PMCID: PMC3314513
- DOI: 10.1124/pr.57.2.7
The molecular pharmacology and cell biology of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors
Abstract
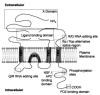
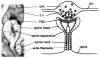
Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors (AMPARs) are of fundamental importance in the brain. They are responsible for the majority of fast excitatory synaptic transmission, and their overactivation is potently excitotoxic. Recent findings have implicated AMPARs in synapse formation and stabilization, and regulation of functional AMPARs is the principal mechanism underlying synaptic plasticity. Changes in AMPAR activity have been described in the pathology of numerous diseases, such as Alzheimer's disease, stroke, and epilepsy. Unsurprisingly, the developmental and activity-dependent changes in the functional synaptic expression of these receptors are under tight cellular regulation. The molecular and cellular mechanisms that control the postsynaptic insertion, arrangement, and lifetime of surface-expressed AMPARs are the subject of intense and widespread investigation. For example, there has been an explosion of information about proteins that interact with AMPAR subunits, and these interactors are beginning to provide real insight into the molecular and cellular mechanisms underlying the cell biology of AMPARs. As a result, there has been considerable progress in this field, and the aim of this review is to provide an account of the current state of knowledge.
Figures



Similar articles
-
D-aspartate and NMDA, but not L-aspartate, block AMPA receptors in rat hippocampal neurons.Br J Pharmacol. 2005 Jun;145(4):449-59. doi: 10.1038/sj.bjp.0706199. Br J Pharmacol. 2005. PMID: 15806114 Free PMC article.
-
Non-fibrillar beta-amyloid abates spike-timing-dependent synaptic potentiation at excitatory synapses in layer 2/3 of the neocortex by targeting postsynaptic AMPA receptors.Eur J Neurosci. 2006 Apr;23(8):2035-47. doi: 10.1111/j.1460-9568.2006.04733.x. Eur J Neurosci. 2006. PMID: 16630051
-
Input- and subunit-specific AMPA receptor trafficking underlying long-term potentiation at hippocampal CA3 synapses.Eur J Neurosci. 2004 Jul;20(1):101-10. doi: 10.1111/j.1460-9568.2004.03461.x. Eur J Neurosci. 2004. PMID: 15245483
-
Regulation of AMPAR trafficking in synaptic plasticity by BDNF and the impact of neurodegenerative disease.J Neurosci Res. 2022 Apr;100(4):979-991. doi: 10.1002/jnr.25022. Epub 2022 Feb 7. J Neurosci Res. 2022. PMID: 35128708 Review.
-
Synaptic plasticity regulated by protein-protein interactions and posttranslational modifications.Int Rev Cell Mol Biol. 2012;297:1-43. doi: 10.1016/B978-0-12-394308-8.00001-7. Int Rev Cell Mol Biol. 2012. PMID: 22608556 Review.
Cited by
-
The dual face of glutamate: from a neurotoxin to a potential survival factor-metabolic implications in health and disease.Cell Mol Life Sci. 2019 Apr;76(8):1473-1488. doi: 10.1007/s00018-018-3002-x. Epub 2019 Jan 1. Cell Mol Life Sci. 2019. PMID: 30599069 Free PMC article. Review.
-
Neuroprotective and anticonvulsant effects of EGIS-8332, a non-competitive AMPA receptor antagonist, in a range of animal models.Br J Pharmacol. 2007 Sep;152(1):151-60. doi: 10.1038/sj.bjp.0707362. Epub 2007 Jul 2. Br J Pharmacol. 2007. PMID: 17603549 Free PMC article.
-
Differential roles of GRIP1a and GRIP1b in AMPA receptor trafficking.Neurosci Lett. 2010 Nov 26;485(3):167-72. doi: 10.1016/j.neulet.2010.09.003. Epub 2010 Sep 17. Neurosci Lett. 2010. PMID: 20837103 Free PMC article.
-
The Effects of Intraoperative Hypothermia on Postoperative Cognitive Function in the Rat Hippocampus and Its Possible Mechanisms.Brain Sci. 2022 Jan 12;12(1):96. doi: 10.3390/brainsci12010096. Brain Sci. 2022. PMID: 35053838 Free PMC article.
-
A juvenile mouse model of anti-N-methyl-D-aspartate receptor encephalitis by active immunization.Front Mol Neurosci. 2023 Sep 18;16:1211119. doi: 10.3389/fnmol.2023.1211119. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37790883 Free PMC article.
References
-
- Akbarian S, Smith MA, Jones EG. Editing for an AMPA receptor subunit RNA in prefrontal cortex and striatum in Alzheimer’s disease, Huntington’s disease and schizophrenia. Brain Res. 1995;699:297–304. - PubMed
-
- Aoki C, Miko I, Oviedo H, Mikeladze-Dvali T, Alexandre L, Sweeney N, Bredt DS. Electron microscopic immunocytochemical detection of PSD-95, PSD-93, SAP-102, and SAP-97 at postsynaptic, presynaptic and nonsynaptic sites of adult and neonatal rat visual cortex. Synapse. 2001;40:239–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
