Opioid antagonists differ according to negative intrinsic efficacy in a mouse model of acute dependence
- PMID: 15912139
- PMCID: PMC1576205
- DOI: 10.1038/sj.bjp.0706247
Opioid antagonists differ according to negative intrinsic efficacy in a mouse model of acute dependence
Abstract
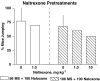
The purpose of the present study is to compare the capacity of opioid antagonists to elicit withdrawal jumping in mice following two acute pretreatment doses of the opioid agonist morphine. Antagonists that precipitate vigorous withdrawal jumping across both morphine treatment doses are hypothesized to be strong inverse agonists at the mu-opioid receptor, whereas antagonists that elicit withdrawal jumping in mice treated with the high but not the low dose of morphine are hypothesized to be weak inverse agonists. Male, Swiss-Webster mice (15-30 g) were acutely treated with 56 or 180 mg kg(-1) morphine 4 h prior to injection with naloxone, naltrexone, diprenorphine, nalorphine, or naloxonazine. Vertical jumping, paw tremors, and weight loss were recorded. Naloxone, naltrexone, and diprenorphine produced withdrawal jumping after 56 and 180 mg kg(-1)morphine pretreatment. Nalorphine and naloxonazine produced moderate withdrawal jumping after 180 mg kg(-1) morphine pretreatment, but failed to elicit significant withdrawal jumping after 56 mg kg(-1) morphine pretreatment. Nalorphine and naloxonazine blocked the withdrawal jumping produced by naloxone. All antagonists produced paw tremors and weight loss although these effects were generally not dose-dependent. Taken together, these findings reveal a rank order of negative intrinsic efficacy for these opioid antagonists as follows: naloxone=naltrexone> or =diprenorphine>nalorphine=naloxonazine. Furthermore, the observation that nalorphine and naloxonazine blocked the naloxone-induced withdrawal jumping provides additional evidence that nalorphine and naloxonazine are weaker inverse agonists than naloxone.
Figures
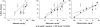
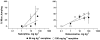
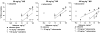
Similar articles
-
Naloxone rapidly evokes endogenous kappa opioid receptor-mediated hyperalgesia in naïve mice pretreated briefly with GM1 ganglioside or in chronic morphine-dependent mice.Brain Res. 2007 Sep 5;1167:31-41. doi: 10.1016/j.brainres.2007.06.058. Epub 2007 Jul 14. Brain Res. 2007. PMID: 17692296
-
Inverse agonists and neutral antagonists at mu opioid receptor (MOR): possible role of basal receptor signaling in narcotic dependence.J Neurochem. 2001 Jun;77(6):1590-600. doi: 10.1046/j.1471-4159.2001.00362.x. J Neurochem. 2001. PMID: 11413242
-
In rats, acute morphine dependence results in antagonist-induced response suppression of intracranial self-stimulation.Psychopharmacology (Berl). 2004 Sep;175(3):287-95. doi: 10.1007/s00213-004-1829-3. Psychopharmacology (Berl). 2004. PMID: 15024547
-
Effects of naloxone and D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 and the protein kinase inhibitors H7 and H8 on acute morphine dependence and antinociceptive tolerance in mice.J Pharmacol Exp Ther. 1996 Apr;277(1):484-90. J Pharmacol Exp Ther. 1996. PMID: 8613958
-
Limitations on the antagonistic actions of opioid antagonists.Fed Proc. 1982 May;41(7):2333-8. Fed Proc. 1982. PMID: 7042396 Review.
Cited by
-
The relative potency of inverse opioid agonists and a neutral opioid antagonist in precipitated withdrawal and antagonism of analgesia and toxicity.J Pharmacol Exp Ther. 2009 Aug;330(2):513-9. doi: 10.1124/jpet.109.152678. Epub 2009 May 12. J Pharmacol Exp Ther. 2009. PMID: 19435929 Free PMC article.
-
Endogenous analgesia, dependence, and latent pain sensitization.Curr Top Behav Neurosci. 2014;20:283-325. doi: 10.1007/7854_2014_351. Curr Top Behav Neurosci. 2014. PMID: 25227929 Free PMC article.
-
Comparison of the opioid receptor antagonist properties of naltrexone and 6 beta-naltrexol in morphine-naïve and morphine-dependent mice.Eur J Pharmacol. 2008 Mar 31;583(1):48-55. doi: 10.1016/j.ejphar.2008.01.004. Epub 2008 Jan 24. Eur J Pharmacol. 2008. PMID: 18275956 Free PMC article.
-
Synthesis and Structure-Activity Relationships of 5'-Aryl-14-alkoxypyridomorphinans: Identification of a μ Opioid Receptor Agonist/δ Opioid Receptor Antagonist Ligand with Systemic Antinociceptive Activity and Diminished Opioid Side Effects.J Med Chem. 2020 Jul 23;63(14):7663-7694. doi: 10.1021/acs.jmedchem.0c00503. Epub 2020 Jun 30. J Med Chem. 2020. PMID: 32530286 Free PMC article.
-
Analgesic tone conferred by constitutively active mu opioid receptors in mice lacking β-arrestin 2.Mol Pain. 2011 Apr 12;7:24. doi: 10.1186/1744-8069-7-24. Mol Pain. 2011. PMID: 21486473 Free PMC article.
References
-
- ADAMS J.U., GELLER E.B., ADLER M.W. Receptor selectivity of icv morphine in the rat cold water tail-flick test. Drug Al. Depend. 1994;35:197–202. - PubMed
-
- BILSKY E.J., BERNSTEIN R.N., WANG Z., SADEE W., PORRECA F. Effects of naloxone and D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 and the protein kinase inhibitors H7 and H8 on acute morphine dependence and antinociceptive tolerance in mice. J. Pharmacol. Exp. Ther. 1996;277:484–490. - PubMed
-
- BLÄSIG J., HERZ A., REINHOLD K., ZIEGLGANSBERGER S. Development of physical dependence on morphine in respect to time and dosage and quantification of the precipitated withdrawal syndrome in rats. Psychopharmacologia. 1973;33:19–38. - PubMed
-
- BLÄSIG J., HÖLLT V., HERZ A., PASCHELKE G. Comparison of withdrawal precipitating properties of various morphine antagonists and partial agonists in relation to their stereospecific binding to brain homogenates. Psychopharmacologia. 1976;46:41–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

