Histone modifications affect timing of oligodendrocyte progenitor differentiation in the developing rat brain
- PMID: 15897262
- PMCID: PMC2171688
- DOI: 10.1083/jcb.200412101
Histone modifications affect timing of oligodendrocyte progenitor differentiation in the developing rat brain
Abstract
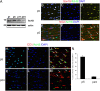
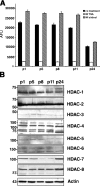
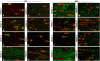
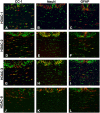
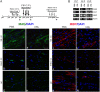
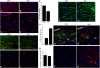
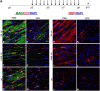
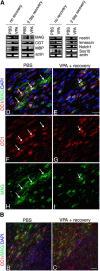
Timely differentiation of progenitor cells is critical for development. In this study we asked whether global epigenetic mechanisms regulate timing of progenitor cell differentiation into myelin-forming oligodendrocytes in vivo. Histone deacetylation was essential during a specific temporal window of development and was dependent on the enzymatic activity of histone deacetylases, whose expression was detected in the developing corpus callosum. During the first 10 postnatal days, administration of valproic acid (VPA), the specific inhibitor for histone deacetylase activity, resulted in significant hypomyelination with delayed expression of late differentiation markers and retained expression of progenitor markers. Differentiation resumed in VPA-injected rats if a recovery period was allowed. Administration of VPA after myelination onset had no effect on myelin gene expression and was consistent with changes of nucleosomal histones from reversible deacetylation to more stable methylation and chromatin compaction. Together, these data identify global modifications of nucleosomal histones critical for timing of oligodendrocyte differentiation and myelination in the developing corpus callosum.
Figures

Similar articles
-
The Autotaxin-Lysophosphatidic Acid Axis Modulates Histone Acetylation and Gene Expression during Oligodendrocyte Differentiation.J Neurosci. 2015 Aug 12;35(32):11399-414. doi: 10.1523/JNEUROSCI.0345-15.2015. J Neurosci. 2015. PMID: 26269646 Free PMC article.
-
Histone deacetylase activity is necessary for oligodendrocyte lineage progression.J Neurosci. 2002 Dec 1;22(23):10333-45. doi: 10.1523/JNEUROSCI.22-23-10333.2002. J Neurosci. 2002. PMID: 12451133 Free PMC article.
-
PRMT5-mediated regulation of developmental myelination.Nat Commun. 2018 Jul 19;9(1):2840. doi: 10.1038/s41467-018-04863-9. Nat Commun. 2018. PMID: 30026560 Free PMC article.
-
Post-translational modifications of nucleosomal histones in oligodendrocyte lineage cells in development and disease.J Mol Neurosci. 2008 May;35(1):13-22. doi: 10.1007/s12031-007-9014-x. J Mol Neurosci. 2008. PMID: 17999198 Free PMC article. Review.
-
CpG island methylation and histone modifications: biology and clinical significance.Ernst Schering Res Found Workshop. 2006;(57):115-26. doi: 10.1007/3-540-37633-x_7. Ernst Schering Res Found Workshop. 2006. PMID: 16568952 Review.
Cited by
-
Intracortical myelination in musicians with absolute pitch: Quantitative morphometry using 7-T MRI.Hum Brain Mapp. 2016 Oct;37(10):3486-501. doi: 10.1002/hbm.23254. Epub 2016 May 10. Hum Brain Mapp. 2016. PMID: 27160707 Free PMC article.
-
The Autotaxin-Lysophosphatidic Acid Axis Modulates Histone Acetylation and Gene Expression during Oligodendrocyte Differentiation.J Neurosci. 2015 Aug 12;35(32):11399-414. doi: 10.1523/JNEUROSCI.0345-15.2015. J Neurosci. 2015. PMID: 26269646 Free PMC article.
-
Epigenetic modulation of seizure-induced neurogenesis and cognitive decline.J Neurosci. 2007 May 30;27(22):5967-75. doi: 10.1523/JNEUROSCI.0110-07.2007. J Neurosci. 2007. PMID: 17537967 Free PMC article.
-
The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation.Neuron. 2007 Jul 19;55(2):217-30. doi: 10.1016/j.neuron.2007.06.029. Neuron. 2007. PMID: 17640524 Free PMC article.
-
Epigenetics and neural stem cell commitment.Neurosci Bull. 2007 Jul;23(4):241-8. doi: 10.1007/s12264-007-0036-8. Neurosci Bull. 2007. PMID: 17687400 Free PMC article. Review.
References
-
- Bannister, A.J., P. Zegerman, J.F. Partridge, E.A. Miska, J.O. Thomas, R.C. Allshire, and T. Kouzarides. 2001. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 410:120–124. - PubMed
-
- Barres, B.A., M.A. Lazar, and M.C. Raff. 1994. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 120:1097–1108. - PubMed
-
- Bjartmar, C., C. Hildebrand, and K. Loinder. 1994. Morphological heterogeneity of rat oligodendrocytes: electron microscopic studies on serial sections. Glia. 11:235–244. - PubMed
-
- Bjelke, B., and A. Seiger. 1989. Morphological distribution of MBP-like immunoreactivity in the brain during development. Int. J. Dev. Neurosci. 7:145–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

