Spatial segregation of gamma-secretase and substrates in distinct membrane domains
- PMID: 15886206
- PMCID: PMC1201532
- DOI: 10.1074/jbc.M503570200
Spatial segregation of gamma-secretase and substrates in distinct membrane domains
Abstract
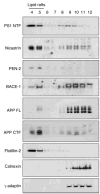
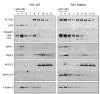
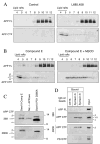
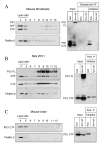
Gamma-secretase facilitates the regulated intramembrane proteolysis of select type I membrane proteins that play diverse physiological roles in multiple cell types and tissue. In this study, we used biochemical approaches to examine the distribution of amyloid precursor protein (APP) and several additional gamma-secretase substrates in membrane microdomains. We report that APP C-terminal fragments (CTFs) and gamma-secretase reside in Lubrol WX detergent-insoluble membranes (DIM) of cultured cells and adult mouse brain. APP CTFs that accumulate in cells lacking gamma-secretase activity preferentially associate with DIM. Cholesterol depletion and magnetic immunoisolation studies indicate recruitment of APP CTFs into cholesterol- and sphingolipid-rich lipid rafts, and co-residence of APP CTFs, PS1, and syntaxin 6 in DIM patches derived from the trans-Golgi network. Photoaffinity cross-linking studies provided evidence for the preponderance of active gamma-secretase in lipid rafts of cultured cells and adult brain. Remarkably, unlike the case of APP, CTFs derived from Notch1, Jagged2, deleted in colorectal cancer (DCC), and N-cadherin remain largely detergent-soluble, indicative of their spatial segregation in non-raft domains. In embryonic brain, the majority of PS1 and nicastrin is present in Lubrol WX-soluble membranes, wherein the CTFs derived from APP, Notch1, DCC, and N-cadherin also reside. We suggest that gamma-secretase residence in non-raft membranes facilitates proteolysis of diverse substrates during embryonic development but that the translocation of gamma-secretase to lipid rafts in adults ensures processing of certain substrates, including APP CTFs, while limiting processing of other potential substrates.
Figures



Similar articles
-
Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes.J Biol Chem. 2004 Oct 22;279(43):44945-54. doi: 10.1074/jbc.M407986200. Epub 2004 Aug 17. J Biol Chem. 2004. PMID: 15322084 Free PMC article.
-
S-palmitoylation of gamma-secretase subunits nicastrin and APH-1.J Biol Chem. 2009 Jan 16;284(3):1373-84. doi: 10.1074/jbc.M806380200. Epub 2008 Nov 20. J Biol Chem. 2009. PMID: 19028695 Free PMC article.
-
A role for presenilin 1 in regulating the delivery of amyloid precursor protein to the cell surface.Neurobiol Dis. 2002 Oct;11(1):64-82. doi: 10.1006/nbdi.2002.0546. Neurobiol Dis. 2002. PMID: 12460547
-
Role of presenilin in gamma-secretase cleavage of amyloid precursor protein.Exp Gerontol. 2000 Jul;35(4):453-60. doi: 10.1016/s0531-5565(00)00111-x. Exp Gerontol. 2000. PMID: 10959033 Review.
-
The role of proteolysis in Alzheimer's disease.Adv Exp Med Biol. 2000;477:379-90. doi: 10.1007/0-306-46826-3_39. Adv Exp Med Biol. 2000. PMID: 10849764 Review.
Cited by
-
ApoER2 expression increases Abeta production while decreasing Amyloid Precursor Protein (APP) endocytosis: Possible role in the partitioning of APP into lipid rafts and in the regulation of gamma-secretase activity.Mol Neurodegener. 2007 Jul 9;2:14. doi: 10.1186/1750-1326-2-14. Mol Neurodegener. 2007. PMID: 17620134 Free PMC article.
-
The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease.Nat Genet. 2007 Feb;39(2):168-77. doi: 10.1038/ng1943. Epub 2007 Jan 14. Nat Genet. 2007. PMID: 17220890 Free PMC article.
-
Deregulated sphingolipid metabolism and membrane organization in neurodegenerative disorders.Mol Neurobiol. 2010 Jun;41(2-3):314-40. doi: 10.1007/s12035-009-8096-6. Epub 2010 Feb 3. Mol Neurobiol. 2010. PMID: 20127207 Review.
-
Mechanisms of amyloid-Beta Peptide uptake by neurons: the role of lipid rafts and lipid raft-associated proteins.Int J Alzheimers Dis. 2010 Dec 20;2011:548380. doi: 10.4061/2011/548380. Int J Alzheimers Dis. 2010. PMID: 21197446 Free PMC article.
-
Persistence of psychosine in brain lipid rafts is a limiting factor in the therapeutic recovery of a mouse model for Krabbe disease.J Neurosci Res. 2011 Mar;89(3):352-64. doi: 10.1002/jnr.22564. Epub 2010 Dec 29. J Neurosci Res. 2011. PMID: 21259322 Free PMC article.
References
-
- Sisodia SS, St George-Hyslop PH. Nat Rev Neurosci. 2002;3:281–290. - PubMed
-
- Vassar R. J Mol Neurosci. 2004;23:105–114. - PubMed
-
- Iwatsubo T. Curr Opin Neurobiol. 2004;14:379–383. - PubMed
-
- Tanzi RE, Bertram L. Neuron. 2001;32:181–184. - PubMed
-
- Levitan D, Greenwald I. Nature. 1995;377:351–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

