Polyamine depletion inhibits NF-kappaB binding to DNA and interleukin-8 production in human chondrocytes stimulated by tumor necrosis factor-alpha
- PMID: 15828019
- PMCID: PMC1226412
- DOI: 10.1002/jcp.20368
Polyamine depletion inhibits NF-kappaB binding to DNA and interleukin-8 production in human chondrocytes stimulated by tumor necrosis factor-alpha
Abstract
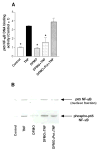
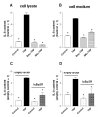
The activation of the NF-kappaB pathway by pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNFalpha), can be an important contributor for the re-programming of chondrocyte gene expression, thereby making it a therapeutic target in articular diseases. To search for new approaches to limit cartilage damage, we investigated the requirement of polyamines for NF-kappaB activation by TNFalpha in human C-28/I2 chondrocytes, using alpha-difluoromethylornithine (DFMO), a specific polyamine biosynthesis inhibitor. The NF-kappaB pathway was dissected by using pharmacological inhibitors or by expressing a transdominant IkappaBalpha super repressor. Treatment of C-28/I2 chondrocytes with TNFalpha resulted in a rapid enhancement of nuclear localization and DNA binding activity of the p65 NF-kappaB subunit. TNFalpha also increased the level and extracellular release of interleukin-8 (IL-8), a CXC chemokine that can have a role in arthritis, in an NF-kappaB-dependent manner. Pre-treatment of chondrocytes with DFMO, while causing polyamine depletion, significantly reduced NF-kappaB DNA binding activity. Moreover, DFMO also decreased IL-8 production without affecting cellular viability. Restoration of polyamine levels by the co-addition of putrescine circumvented the inhibitory effects of DFMO. Our results show that the intracellular depletion of polyamines inhibits the response of chondrocytes to TNFalpha by interfering with the DNA binding activity of NF-kappaB. This suggests that a pharmacological and/or genetic approach to deplete the polyamine pool in chondrocytes may represent a useful way to reduce NF-kappaB activation by inflammatory cytokines in arthritis without provoking chondrocyte apoptosis.
Copyright 2005 Wiley-Liss, Inc.
Figures
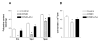
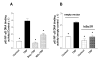
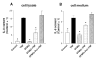
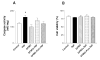
Similar articles
-
Polyamine depletion inhibits apoptosis following blocking of survival pathways in human chondrocytes stimulated by tumor necrosis factor-alpha.J Cell Physiol. 2006 Jan;206(1):138-46. doi: 10.1002/jcp.20446. J Cell Physiol. 2006. PMID: 15965903
-
NF-kappaB activation and susceptibility to apoptosis after polyamine depletion in intestinal epithelial cells.Am J Physiol Gastrointest Liver Physiol. 2001 May;280(5):G992-G1004. doi: 10.1152/ajpgi.2001.280.5.G992. Am J Physiol Gastrointest Liver Physiol. 2001. PMID: 11292609
-
Interleukin-1β and tumor necrosis factor-α augment acidosis-induced rat articular chondrocyte apoptosis via nuclear factor-kappaB-dependent upregulation of ASIC1a channel.Biochim Biophys Acta Mol Basis Dis. 2018 Jan;1864(1):162-177. doi: 10.1016/j.bbadis.2017.10.004. Epub 2017 Oct 3. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 28986307
-
NF-κB Signaling Regulates Physiological and Pathological Chondrogenesis.Int J Mol Sci. 2019 Dec 12;20(24):6275. doi: 10.3390/ijms20246275. Int J Mol Sci. 2019. PMID: 31842396 Free PMC article. Review.
-
Cachexia: a therapeutic approach beyond cytokine antagonism.Int J Cardiol. 2002 Sep;85(1):173-83. doi: 10.1016/s0167-5273(02)00245-0. Int J Cardiol. 2002. PMID: 12163222 Review.
Cited by
-
Differential requirements for IKKalpha and IKKbeta in the differentiation of primary human osteoarthritic chondrocytes.Arthritis Rheum. 2008 Jan;58(1):227-39. doi: 10.1002/art.23211. Arthritis Rheum. 2008. PMID: 18163512 Free PMC article.
-
Matrix metalloproteinase 13 loss associated with impaired extracellular matrix remodeling disrupts chondrocyte differentiation by concerted effects on multiple regulatory factors.Arthritis Rheum. 2010 Aug;62(8):2370-81. doi: 10.1002/art.27512. Arthritis Rheum. 2010. PMID: 20506238 Free PMC article.
-
Biochemical and proteomic characterization of alkaptonuric chondrocytes.J Cell Physiol. 2012 Sep;227(9):3333-43. doi: 10.1002/jcp.24033. J Cell Physiol. 2012. PMID: 22213341 Free PMC article.
-
Sustained NF-kappaB activation produces a short-term cell proliferation block in conjunction with repressing effectors of cell cycle progression controlled by E2F or FoxM1.J Cell Physiol. 2009 Jan;218(1):215-27. doi: 10.1002/jcp.21596. J Cell Physiol. 2009. PMID: 18803232 Free PMC article.
-
Antioxidants inhibit SAA formation and pro-inflammatory cytokine release in a human cell model of alkaptonuria.Rheumatology (Oxford). 2013 Sep;52(9):1667-73. doi: 10.1093/rheumatology/ket185. Epub 2013 May 23. Rheumatology (Oxford). 2013. PMID: 23704321 Free PMC article.
References
-
- Aigner T, Kim HA. Apoptosis and cellular vitality: issues in osteoarthritic cartilage degeneration. Arthritis Rheum. 2002;46(8):1986–1996. - PubMed
-
- Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression. Nature. 2003;423(6940):659–663. - PubMed
-
- Bachrach U, Wang YC, Tabib A. Polyamines: new cues in cellular signal transduction. News Physiol Sci. 2001;16:106–109. - PubMed
-
- Borzi RM, Mazzetti I, Cattini L, Uguccioni M, Baggiolini M, Facchini A. Human chondrocytes express functional chemokine receptors and release matrix-degrading enzymes in response to C-X-C and C-C chemokines. Arthritis Rheum. 2000;43(8):1734–1741. - PubMed
-
- Borzi RM, Mazzetti I, Macor S, Silvestri T, Bassi A, Cattini L, Facchini A. Flow cytometric analysis of intracellular chemokines in chondrocytes in vivo: constitutive expression and enhancement in osteoarthritis and rheumatoid arthritis. FEBS Lett. 1999;455(3):238–242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

