Regulation of translation by ribosome shunting through phosphotyrosine-dependent coupling of adenovirus protein 100k to viral mRNAs
- PMID: 15827182
- PMCID: PMC1082770
- DOI: 10.1128/JVI.79.9.5676-5683.2005
Regulation of translation by ribosome shunting through phosphotyrosine-dependent coupling of adenovirus protein 100k to viral mRNAs
Abstract
Adenovirus simultaneously inhibits cap-dependent host cell mRNA translation while promoting the translation of its late viral mRNAs during infection. Studies previously demonstrated that tyrosine kinase activity plays a central role in the control of late adenovirus protein synthesis. The tyrosine kinase inhibitor genistein decreases late viral mRNA translation and prevents viral inhibition of cellular protein synthesis. Adenovirus protein 100k blocks cellular mRNA translation by disrupting the cap-initiation complex and promotes viral mRNA translation through an alternate mechanism known as ribosome shunting. 100k protein interaction with initiation factor eIF4G and the viral 5' noncoding region on viral late mRNAs, known as the tripartite leader, are both essential for ribosome shunting. We show that adenovirus protein 100k promotes ribosome shunting in a tyrosine phosphorylation-dependent manner. The primary sites of phosphorylated tyrosine on protein 100k were mapped and mutated, and two key sites are shown to be essential for protein 100k to promote ribosome shunting. Mutation of the two tyrosine phosphorylation sites in 100k protein does not impair interaction with initiation factor 4G, but it severely reduces association of 100k with tripartite leader mRNAs. 100k protein therefore promotes ribosome shunting and selective translation of viral mRNAs by binding specifically to the adenovirus tripartite leader in a phosphotyrosine-dependent manner.
Figures

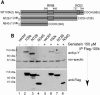
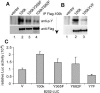

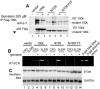

Similar articles
-
Tethering of eIF4G to adenoviral mRNAs by viral 100k protein drives ribosome shunting.Genes Dev. 2004 Aug 15;18(16):1997-2009. doi: 10.1101/gad.1212504. Genes Dev. 2004. PMID: 15314025 Free PMC article.
-
Structural basis for competitive inhibition of eIF4G-Mnk1 interaction by the adenovirus 100-kilodalton protein.J Virol. 2004 Jul;78(14):7707-16. doi: 10.1128/JVI.78.14.7707-7716.2004. J Virol. 2004. PMID: 15220445 Free PMC article.
-
Internal initiation of translation mediated by the 5' leader of a cellular mRNA.Nature. 1991 Sep 5;353(6339):90-4. doi: 10.1038/353090a0. Nature. 1991. PMID: 1652694
-
Structural and mechanistic insights into hepatitis C viral translation initiation.Nat Rev Microbiol. 2007 Jan;5(1):29-38. doi: 10.1038/nrmicro1558. Epub 2006 Nov 27. Nat Rev Microbiol. 2007. PMID: 17128284 Review.
-
Poliovirus translation: a paradigm for a novel initiation mechanism.Bioessays. 1989 Nov;11(5):128-32. doi: 10.1002/bies.950110504. Bioessays. 1989. PMID: 2556117 Review.
Cited by
-
The Human Adenovirus Type 2 Transcriptome: An Amazing Complexity of Alternatively Spliced mRNAs.J Virol. 2021 Feb 15;95(4):e01869-20. doi: 10.1128/JVI.01869-20. Epub 2020 Nov 25. J Virol. 2021. PMID: 33239457 Free PMC article.
-
CRM1-dependent transport supports cytoplasmic accumulation of adenoviral early transcripts.J Virol. 2012 Feb;86(4):2282-92. doi: 10.1128/JVI.06275-11. Epub 2011 Dec 14. J Virol. 2012. PMID: 22171254 Free PMC article.
-
Translation initiation of viral mRNAs.Rev Med Virol. 2010 May;20(3):177-95. doi: 10.1002/rmv.649. Rev Med Virol. 2010. PMID: 20440748 Free PMC article. Review.
-
Noncytotoxic functions of killer cell granzymes in viral infections.PLoS Pathog. 2021 Sep 16;17(9):e1009818. doi: 10.1371/journal.ppat.1009818. eCollection 2021 Sep. PLoS Pathog. 2021. PMID: 34529743 Free PMC article. Review.
-
Adenovirus ubiquitin-protein ligase stimulates viral late mRNA nuclear export.J Virol. 2007 Jan;81(2):575-87. doi: 10.1128/JVI.01725-06. Epub 2006 Nov 1. J Virol. 2007. PMID: 17079297 Free PMC article.
References
-
- Akiyama, T., J. Ishida, S. Nakagawa, H. Ogawara, S. Watanabe, N. Itoh, M. Shibuya, and Y. Fukami. 1987. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 262:5592-5595. - PubMed
-
- Bablanian, R., and W. C. Russell. 1974. Adenovirus polypeptide synthesis in the presence of non-replicating poliovirus. J. Gen. Virol. 24:261-279. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

