The nucleotide switch in Cdc42 modulates coupling between the GTPase-binding and allosteric equilibria of Wiskott-Aldrich syndrome protein
- PMID: 15821030
- PMCID: PMC556282
- DOI: 10.1073/pnas.0406472102
The nucleotide switch in Cdc42 modulates coupling between the GTPase-binding and allosteric equilibria of Wiskott-Aldrich syndrome protein
Abstract
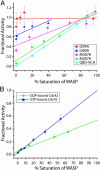
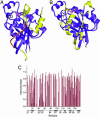
The GTP/GDP nucleotide switch in Ras superfamily GTPases generally involves differential affinity toward downstream effectors, with the GTP-bound state having a higher affinity for effector than the GDP-bound state. We have developed a quantitative model of allosteric regulation of the Wiskott-Aldrich syndrome protein (WASP) by the Rho GTPase Cdc42 to better understand how GTPase binding is coupled to effector activation. The model accurately predicts WASP affinity for Cdc42, activity toward Arp2/3 complex, and activation by Cdc42 as functions of a two-state allosteric equilibrium in WASP. The ratio of GTPase affinities for the inactive and active states of WASP is appreciably larger for Cdc42-GTP than for Cdc42-GDP. The greater ability to distinguish between the two states of WASP makes Cdc42-GTP a full WASP agonist, whereas Cdc42-GDP is only a partial agonist. Thus, the nucleotide switch controls not only the affinity of Cdc42 for its effector but also the efficiency of coupling between the Cdc42-binding and allosteric equilibria in WASP. This effect can ensure high fidelity and specificity in Cdc42 signaling in crowded membrane environments.
Figures
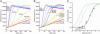

Similar articles
-
Structure of Cdc42 in complex with the GTPase-binding domain of the 'Wiskott-Aldrich syndrome' protein.Nature. 1999 May 27;399(6734):379-83. doi: 10.1038/20726. Nature. 1999. PMID: 10360578
-
Global disruption of the WASP autoinhibited structure on Cdc42 binding. Ligand displacement as a novel method for monitoring amide hydrogen exchange.Biochemistry. 2001 Nov 27;40(47):14115-22. doi: 10.1021/bi0157215. Biochemistry. 2001. PMID: 11714264
-
The adapter protein CrkII regulates neuronal Wiskott-Aldrich syndrome protein, actin polymerization, and tension development during contractile stimulation of smooth muscle.J Biol Chem. 2005 Jun 17;280(24):23380-9. doi: 10.1074/jbc.M413390200. Epub 2005 Apr 17. J Biol Chem. 2005. PMID: 15834156
-
Wiskott-Aldrich syndrome: a disorder of haematopoietic cytoskeletal regulation.Microsc Res Tech. 1999 Oct 15;47(2):107-13. doi: 10.1002/(SICI)1097-0029(19991015)47:2<107::AID-JEMT3>3.0.CO;2-H. Microsc Res Tech. 1999. PMID: 10523789 Review.
-
Cdc42 in actin dynamics: An ordered pathway governed by complex equilibria and directional effector handover.Small GTPases. 2017 Oct 2;8(4):237-244. doi: 10.1080/21541248.2016.1215657. Epub 2016 Aug 11. Small GTPases. 2017. PMID: 27715449 Free PMC article. Review.
Cited by
-
Arp2/3-independent assembly of actin by Vibrio type III effector VopL.Proc Natl Acad Sci U S A. 2007 Oct 23;104(43):17117-22. doi: 10.1073/pnas.0703196104. Epub 2007 Oct 17. Proc Natl Acad Sci U S A. 2007. PMID: 17942696 Free PMC article.
-
Allosteric cooperativity in protein kinase A.Proc Natl Acad Sci U S A. 2008 Jan 15;105(2):506-11. doi: 10.1073/pnas.0709214104. Epub 2008 Jan 4. Proc Natl Acad Sci U S A. 2008. PMID: 18178622 Free PMC article.
-
Visualizing dynamic interaction between calmodulin and calmodulin-related kinases via a monitoring method in live mammalian cells.Proc Natl Acad Sci U S A. 2010 Feb 23;107(8):3412-7. doi: 10.1073/pnas.0911262107. Epub 2010 Feb 3. Proc Natl Acad Sci U S A. 2010. PMID: 20133723 Free PMC article.
-
On the supertertiary structure of proteins.Nat Chem Biol. 2012 Jun 18;8(7):597-600. doi: 10.1038/nchembio.1009. Nat Chem Biol. 2012. PMID: 22710296 No abstract available.
-
Can membrane composition traffic toxins? Mycolactone and preferential membrane interactions.Biophys J. 2022 Nov 15;121(22):4260-4270. doi: 10.1016/j.bpj.2022.10.019. Epub 2022 Oct 18. Biophys J. 2022. PMID: 36258678 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

