Androgen receptor signaling is required for androgen-sensitive human prostate cancer cell proliferation and survival
- PMID: 15813967
- PMCID: PMC1087496
- DOI: 10.1186/1475-2867-5-8
Androgen receptor signaling is required for androgen-sensitive human prostate cancer cell proliferation and survival
Abstract
BACKGROUND: Androgens and androgen receptors (AR) regulate normal prostate development and growth. They also are involved in pathological development of prostatic diseases, including benign prostatic hyperplasia (BPH) and prostate cancer (PCa). Antiandrogen therapy for PCa, in conjunction with chemical or surgical castration, offers initial positive responses and leads to massive prostate cell death. However, cancer cells later appear as androgen-independent PCa. To investigate the role of AR in prostate cell proliferation and survival, we introduced a vector-based small interfering RNA (siRNA). This siRNA targeted 5'-untranslated region of AR mRNA for extended suppression of AR expression in androgen-sensitive human prostate LNCaP cells. RESULTS: The siRNA design successfully suppressed endogenous AR expression, as revealed by western blotting and immunofluorescence staining in LNCaP cells. LNCaP cells did not proliferate in the absence of AR and underwent apoptosis, based on elevated phospho-Histone H2B expression and higher number of apoptotic body as compared to control cells. CONCLUSION: We demonstrated that AR is vital for prostate cell proliferation and survival in this androgen-sensitive prostate cell line. These results further strengthen the hypothesis that AR can be a therapeutic target for treating androgen-sensitive stages of PCa. Unlike antiandorgens, however, siRNA targeting AR provides a direct inactivation of AR function through the suppression of AR protein expression.
Figures

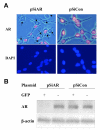
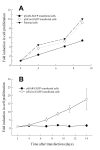
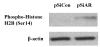
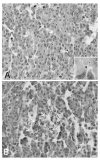
Similar articles
-
The prostate cancer-up-regulated long noncoding RNA PlncRNA-1 modulates apoptosis and proliferation through reciprocal regulation of androgen receptor.Urol Oncol. 2013 Oct;31(7):1117-23. doi: 10.1016/j.urolonc.2011.11.030. Epub 2012 Jan 20. Urol Oncol. 2013. PMID: 22264502
-
Androgens induce a distinct response of epithelial-mesenchymal transition factors in human prostate cancer cells.Mol Cell Biochem. 2016 Oct;421(1-2):139-47. doi: 10.1007/s11010-016-2794-y. Epub 2016 Aug 25. Mol Cell Biochem. 2016. PMID: 27562825
-
Androgen receptor as a regulator of ZEB2 expression and its implications in epithelial-to-mesenchymal transition in prostate cancer.Endocr Relat Cancer. 2014 May 8;21(3):473-86. doi: 10.1530/ERC-13-0514. Print 2014 Jun. Endocr Relat Cancer. 2014. PMID: 24812058
-
The role of the androgen receptor in the development of prostatic hyperplasia and prostate cancer.Mol Cell Biochem. 2003 Nov;253(1-2):89-101. doi: 10.1023/a:1026057402945. Mol Cell Biochem. 2003. PMID: 14619959 Review.
-
Androgen receptors in hormone-dependent and castration-resistant prostate cancer.Pharmacol Ther. 2013 Dec;140(3):223-38. doi: 10.1016/j.pharmthera.2013.07.003. Epub 2013 Jul 13. Pharmacol Ther. 2013. PMID: 23859952 Review.
Cited by
-
Coprinus comatus and Ganoderma lucidum interfere with androgen receptor function in LNCaP prostate cancer cells.Mol Biol Rep. 2008 Jun;35(2):107-17. doi: 10.1007/s11033-007-9059-5. Epub 2007 Mar 13. Mol Biol Rep. 2008. PMID: 17431821
-
Integration of regulatory networks by NKX3-1 promotes androgen-dependent prostate cancer survival.Mol Cell Biol. 2012 Jan;32(2):399-414. doi: 10.1128/MCB.05958-11. Epub 2011 Nov 14. Mol Cell Biol. 2012. PMID: 22083957 Free PMC article.
-
Agent-based modeling of the prostate tumor microenvironment uncovers spatial tumor growth constraints and immunomodulatory properties.NPJ Syst Biol Appl. 2024 Feb 21;10(1):20. doi: 10.1038/s41540-024-00344-6. NPJ Syst Biol Appl. 2024. PMID: 38383542 Free PMC article.
-
Antioxidants Abrogate Alpha-Tocopherylquinone-Mediated Down-Regulation of the Androgen Receptor in Androgen-Responsive Prostate Cancer Cells.PLoS One. 2016 Mar 17;11(3):e0151525. doi: 10.1371/journal.pone.0151525. eCollection 2016. PLoS One. 2016. PMID: 26986969 Free PMC article.
-
Prostate cancer cells tolerate a narrow range of androgen receptor expression and activity.Prostate. 2007 Dec 1;67(16):1801-15. doi: 10.1002/pros.20662. Prostate. 2007. PMID: 17935158 Free PMC article.
References
-
- Culig Z, Hobisch A, Cronauer MV, Cato ACB, Hittmair A, Radmayr C, Eberle J, Bartsch G, Klocker H. Mutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgens and progesterone. Mol Endocrinol. 1993;7:1541–1550. doi: 10.1210/me.7.12.1541. - DOI - PubMed
-
- Buchanan G, Yang M, Harris JM, Nahm HS, Han G, Moore N, Bentel JM, Matusik RJ, Horsfall DJ, Marshall VR, Greenberg NM, Tilley WD. Mutations at the boundary of the hinge and ligand binding domain of the androgen receptor confer increased transactivation function. Mol Endocrinol. 2001;15:46–56. doi: 10.1210/me.15.1.46. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

