Three distinct kinetic groupings of the synaptotagmin family: candidate sensors for rapid and delayed exocytosis
- PMID: 15793006
- PMCID: PMC556003
- DOI: 10.1073/pnas.0500941102
Three distinct kinetic groupings of the synaptotagmin family: candidate sensors for rapid and delayed exocytosis
Erratum in
-
Correction for Hui et al., Three distinct kinetic groupings of the synaptotagmin family: Candidate sensors for rapid and delayed exocytosis.Proc Natl Acad Sci U S A. 2024 Jan 16;121(3):e2321505121. doi: 10.1073/pnas.2321505121. Epub 2024 Jan 12. Proc Natl Acad Sci U S A. 2024. PMID: 38215188 Free PMC article. No abstract available.
Abstract
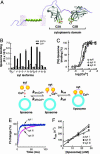
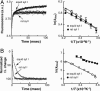
Synaptotagmins (syts) are a family of membrane proteins present on a variety of intracellular organelles. In vertebrates, 16 isoforms of syt have been identified. The most abundant isoform, syt I, appears to function as a Ca2+ sensor that triggers the rapid exocytosis of synaptic vesicles from neurons. The functions of the remaining syt isoforms are less well understood. The cytoplasmic domain of syt I binds membranes in response to Ca2+, and this interaction has been proposed to play a key role in secretion. Here, we tested the Ca(2+)-triggered membrane-binding activity of the cytoplasmic domains of syts I-XII; eight isoforms tightly bound to liposomes that contained phosphatidylserine as a function of the concentration of Ca2+. We then compared the disassembly kinetics of Ca2+.syt.membrane complexes upon rapid mixing with excess Ca2+ chelator and found that syts can be classified into three distinct kinetic groups. syts I, II, and III constitute the fast group; syts V, VI, IX, and X make up the medium group; and syt VII exhibits the slowest kinetics of disassembly. Thus, isoforms of syt, which have much slower disassembly kinetics than does syt I, might function as Ca2+ sensors for asynchronous release, which occurs after Ca2+ domains have collapsed. We also compared the temperature dependence of Ca2+.syt.membrane assembly and disassembly reactions by using squid and rat syt I. These results indicate that syts have diverged to release Ca2+ and membranes with distinct kinetics.
Figures
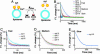
Similar articles
-
Calcium-dependent and -independent hetero-oligomerization in the synaptotagmin family.J Biochem. 2000 Oct;128(4):637-45. doi: 10.1093/oxfordjournals.jbchem.a022796. J Biochem. 2000. PMID: 11011146
-
Ca(2+)-dependent and -independent activities of neural and non-neural synaptotagmins.Nature. 1995 Jun 15;375(6532):594-9. doi: 10.1038/375594a0. Nature. 1995. PMID: 7791877
-
Synaptotagmin V is targeted to dense-core vesicles that undergo calcium-dependent exocytosis in PC12 cells.J Biol Chem. 2002 Jul 5;277(27):24499-505. doi: 10.1074/jbc.M202767200. Epub 2002 May 2. J Biol Chem. 2002. PMID: 12006594
-
Synaptotagmin regulates mast cell functions.Immunol Rev. 2001 Feb;179:25-34. doi: 10.1034/j.1600-065x.2001.790103.x. Immunol Rev. 2001. PMID: 11292024 Review.
-
Role of synaptotagmin, a Ca2+ and inositol polyphosphate binding protein, in neurotransmitter release and neurite outgrowth.Chem Phys Lipids. 1999 Apr;98(1-2):59-67. doi: 10.1016/s0009-3084(99)00018-3. Chem Phys Lipids. 1999. PMID: 10358928 Review.
Cited by
-
Two GABAA responses with distinct kinetics in a sound localization circuit.J Physiol. 2012 Aug 15;590(16):3787-805. doi: 10.1113/jphysiol.2012.230136. Epub 2012 May 21. J Physiol. 2012. PMID: 22615438 Free PMC article.
-
Presynaptic nanodomains: a tale of two synapses.Front Cell Neurosci. 2015 Jan 26;8:455. doi: 10.3389/fncel.2014.00455. eCollection 2014. Front Cell Neurosci. 2015. PMID: 25674049 Free PMC article. Review.
-
Ca(2+) influx and neurotransmitter release at ribbon synapses.Cell Calcium. 2012 Sep-Oct;52(3-4):208-16. doi: 10.1016/j.ceca.2012.06.004. Epub 2012 Jul 8. Cell Calcium. 2012. PMID: 22776680 Free PMC article.
-
The functional significance of synaptotagmin diversity in neuroendocrine secretion.Front Endocrinol (Lausanne). 2013 Sep 18;4:124. doi: 10.3389/fendo.2013.00124. Front Endocrinol (Lausanne). 2013. PMID: 24065953 Free PMC article. Review.
-
Phosphatidylinositol 4,5-bisphosphate drives Ca2+-independent membrane penetration by the tandem C2 domain proteins synaptotagmin-1 and Doc2β.J Biol Chem. 2019 Jul 12;294(28):10942-10953. doi: 10.1074/jbc.RA119.007929. Epub 2019 May 30. J Biol Chem. 2019. PMID: 31147445 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

