Serum-free generation and quantification of functionally active Leukemia-derived DC is possible from malignant blasts in acute myeloid leukemia and myelodysplastic syndromes
- PMID: 15789235
- PMCID: PMC11032985
- DOI: 10.1007/s00262-004-0657-y
Serum-free generation and quantification of functionally active Leukemia-derived DC is possible from malignant blasts in acute myeloid leukemia and myelodysplastic syndromes
Abstract
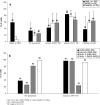
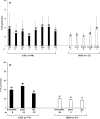
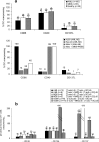
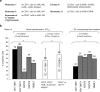
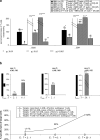
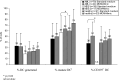
Functional dendritic cells (DC) are professional antigen presenting cells (APC) and can be generated in vitro from leukemic cells from acute myeloid leukemia AML patients, giving rise to APC of leukemic origin presenting leukemic antigens (DC(leu)). We have already shown that DC can be successfully generated from AML and myeloplastic syndromes (MDS) cells in serum-free 'standard' medium (X-vivo + GM-CSF + IL-4 +TNFalpha + FL) in 10-14 days. In this study, we present that DC counts generated from mononuclear cells (MNC) varied between 20% (from 55 MDS samples), 34% (from 100 AML samples) and 25% (from 38 healthy MNC samples) medium. Between 53% and 58% of DC are mature CD83+ DC. DC harvests were highest in monocytoid FAB types (AML-M4/M5, MDS-CMML) and independent from cytogenetic risk groups, demonstrating that DC-based strategies can be applied for patients with all cytogenetic risk groups. Proof of the clonal derivation of DC generated was obtained in five AML and four MDS cases with a combined FISH/immunophenotype analysis (FISH-IPA): The clonal numerical chromosome aberrations of the diseases were regularly codetectable with DC markers; however, not with all clonal cells being convertible to leukemia-derived DC(leu) (on average, 53% of blasts in AML or MDS). To the contrary, not all DC generated carried the clonal aberration (on average, 51% of DC). In 41 AML and 13 MDS cases with a suitable antigen expression, we could confirm FISH-IPA data by Flow cytometry: although DC(leu) are regularly detectable, on average only 57% of blasts in AML and 64% of blasts in MDS were converted to DC(leu). After coculture with DC in mixed lymphocyte reactions (MLR), autologous T cells from AML and MDS patients proliferate and upregulate costimulatory receptors. The specific lysis of leukemic cells by autologous T cells could be demonstrated in three cases with AML in a Fluorolysis assay. In six cases with only few DC(leu) or few vital T cells available after the DC/MLR procedure, no lysis of allogeneic or autologous leukemic cells was seen, pointing to the crucial role of both partners in the lysis process. We conclude: (1) the generation of DC is regularly possible in AML and also in MDS under serum-free conditions. (2) Clonal/leukemia-derived DC(leu) can be regularly generated from MDS and AML-MNC; however, not with all blasts being converted to DC(leu) and not all DC generated carrying leukemic markers. We recommend to select DC(leu) for vaccinations or ex vivo T-cell activations to avoid contaminations with non-converted blasts and non-leukemia-derived DC and to improve the harvest of specific, anti-leukemic T cells. DC and DC-primed T cells could provide a practical strategy for the immunotherapy of AML and MDS.
Figures


Similar articles
-
Leukaemia-derived dendritic cells can be generated from blood or bone marrow cells from patients with myelodysplasia: a methodological approach under serum-free culture conditions.Scand J Immunol. 2005 Jul;62(1):75-85. doi: 10.1111/j.1365-3083.2005.01631.x. Scand J Immunol. 2005. PMID: 16091127
-
Leukemia-derived dendritic cells can be generated from blood or bone marrow cells from patients with acute myeloid leukaemia: a methodological approach under serum-free culture conditions.Scand J Immunol. 2005 Jul;62(1):86-98. doi: 10.1111/j.1365-3083.2005.01630.x. Scand J Immunol. 2005. PMID: 16091128
-
Dendritic cells (DCs) can be successfully generated from leukemic blasts in individual patients with AML or MDS: an evaluation of different methods.J Immunother. 2010 Feb-Mar;33(2):185-99. doi: 10.1097/CJI.0b013e3181b8f4ce. J Immunother. 2010. PMID: 20139775
-
Current Insights into CAR T-Cell-Based Therapies for Myelodysplastic Syndrome.Pharm Res. 2024 Sep;41(9):1757-1773. doi: 10.1007/s11095-024-03761-8. Epub 2024 Aug 26. Pharm Res. 2024. PMID: 39187686 Review.
-
Targeted immunotherapy in acute myeloblastic leukemia: from animals to humans.Cancer Immunol Immunother. 2005 Oct;54(10):933-43. doi: 10.1007/s00262-005-0678-1. Epub 2005 May 12. Cancer Immunol Immunother. 2005. PMID: 15889256 Free PMC article. Review.
Cited by
-
Microenvironmental Features Driving Immune Evasion in Myelodysplastic Syndromes and Acute Myeloid Leukemia.Diseases. 2022 Jun 10;10(2):33. doi: 10.3390/diseases10020033. Diseases. 2022. PMID: 35735633 Free PMC article. Review.
-
The characterization and role of leukemia cell-derived dendritic cells in immunotherapy for leukemic diseases.Intractable Rare Dis Res. 2012 May;1(2):53-65. doi: 10.5582/irdr.2012.v1.2.53. Intractable Rare Dis Res. 2012. PMID: 25343074 Free PMC article. Review.
-
Immunotherapy of myeloid leukaemia.Cancer Immunol Immunother. 2007 Jul;56(7):943-57. doi: 10.1007/s00262-006-0267-y. Epub 2006 Dec 20. Cancer Immunol Immunother. 2007. PMID: 17180671 Free PMC article. Review.
-
Dendritic Cells of Leukemic Origin: Specialized Antigen-Presenting Cells as Potential Treatment Tools for Patients with Myeloid Leukemia.Transfus Med Hemother. 2020 Dec;47(6):432-443. doi: 10.1159/000512452. Epub 2020 Nov 5. Transfus Med Hemother. 2020. PMID: 33442338 Free PMC article. Review.
-
An immune edited tumour versus a tumour edited immune system: Prospects for immune therapy of acute myeloid leukaemia.Cancer Immunol Immunother. 2006 Aug;55(8):1017-24. doi: 10.1007/s00262-006-0129-7. Epub 2006 Feb 1. Cancer Immunol Immunother. 2006. PMID: 16450142 Free PMC article. Review.
References
-
- von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343(14):1020–1034. - PubMed
-
- Appelbaum FR. New targets for therapy in acute myeloid leukemia. Leukemia. 2003;17(3):492–495. - PubMed
-
- Aul C, Giagounidis A, Germing U, Ganser A. Myelodysplastic syndromes. Diagnosis and therapeutic strategies. Med Klin. 2002;97(11):666–676. - PubMed
-
- Balaian L, Ball ED. Direct effect of bispecific anti-CD33×anti-CD64 antibody on proliferation and signaling in myeloid cells. Leuk Res. 2001;25(12):1115–1125. - PubMed
-
- Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976;33(4):451–458. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

