An essential Drosophila glutamate receptor subunit that functions in both central neuropil and neuromuscular junction
- PMID: 15788777
- PMCID: PMC2194804
- DOI: 10.1523/JNEUROSCI.4201-04.2005
An essential Drosophila glutamate receptor subunit that functions in both central neuropil and neuromuscular junction
Abstract
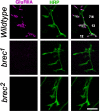
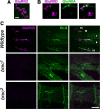
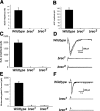
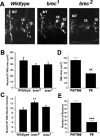
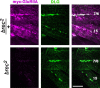
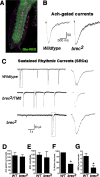
A Drosophila forward genetic screen for mutants with defective synaptic development identified bad reception (brec). Homozygous brec mutants are embryonic lethal, paralyzed, and show no detectable synaptic transmission at the glutamatergic neuromuscular junction (NMJ). Genetic mapping, complementation tests, and genomic sequencing show that brec mutations disrupt a previously uncharacterized ionotropic glutamate receptor subunit, named here "GluRIID." GluRIID is expressed in the postsynaptic domain of the NMJ, as well as widely throughout the synaptic neuropil of the CNS. In the NMJ of null brec mutants, all known glutamate receptor subunits are undetectable by immunocytochemistry, and all functional glutamate receptors are eliminated. Thus, we conclude that GluRIID is essential for the assembly and/or stabilization of glutamate receptors in the NMJ. In null brec mutant embryos, the frequency of periodic excitatory currents in motor neurons is significantly reduced, demonstrating that CNS motor pattern activity is regulated by GluRIID. Although synaptic development and molecular differentiation appear otherwise unperturbed in null mutants, viable hypomorphic brec mutants display dramatically undergrown NMJs by the end of larval development, suggesting that GluRIID-dependent central pattern activity regulates peripheral synaptic growth. These studies reveal GluRIID as a newly identified glutamate receptor subunit that is essential for glutamate receptor assembly/stabilization in the peripheral NMJ and required for properly patterned motor output in the CNS.
Figures


Similar articles
-
Four different subunits are essential for expressing the synaptic glutamate receptor at neuromuscular junctions of Drosophila.J Neurosci. 2005 Mar 23;25(12):3209-18. doi: 10.1523/JNEUROSCI.4194-04.2005. J Neurosci. 2005. PMID: 15788778 Free PMC article.
-
Rolling blackout is required for synaptic vesicle exocytosis.J Neurosci. 2006 Mar 1;26(9):2369-79. doi: 10.1523/JNEUROSCI.3770-05.2006. J Neurosci. 2006. PMID: 16510714 Free PMC article.
-
Postsynaptic actin regulates active zone spacing and glutamate receptor apposition at the Drosophila neuromuscular junction.Mol Cell Neurosci. 2014 Jul;61:241-54. doi: 10.1016/j.mcn.2014.07.005. Epub 2014 Jul 24. Mol Cell Neurosci. 2014. PMID: 25066865 Free PMC article.
-
Development and plasticity of the Drosophila larval neuromuscular junction.Wiley Interdiscip Rev Dev Biol. 2013 Sep-Oct;2(5):647-70. doi: 10.1002/wdev.108. Epub 2013 Feb 5. Wiley Interdiscip Rev Dev Biol. 2013. PMID: 24014452 Free PMC article. Review.
-
Glutamate receptors in synaptic assembly and plasticity: case studies on fly NMJs.Adv Exp Med Biol. 2012;970:3-28. doi: 10.1007/978-3-7091-0932-8_1. Adv Exp Med Biol. 2012. PMID: 22351049 Review.
Cited by
-
Nonvesicular release of glutamate by glial xCT transporters suppresses glutamate receptor clustering in vivo.J Neurosci. 2007 Jan 3;27(1):111-23. doi: 10.1523/JNEUROSCI.4770-06.2007. J Neurosci. 2007. PMID: 17202478 Free PMC article.
-
A circuit-dependent ROS feedback loop mediates glutamate excitotoxicity to sculpt the Drosophila motor system.Elife. 2019 Jul 18;8:e47372. doi: 10.7554/eLife.47372. Elife. 2019. PMID: 31318331 Free PMC article.
-
Glia in Drosophila behavior.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015 Sep;201(9):879-93. doi: 10.1007/s00359-014-0952-9. Epub 2014 Oct 22. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015. PMID: 25336160 Review.
-
Synapse-specific and compartmentalized expression of presynaptic homeostatic potentiation.Elife. 2018 Apr 5;7:e34338. doi: 10.7554/eLife.34338. Elife. 2018. PMID: 29620520 Free PMC article.
-
LRRK2 regulates retrograde synaptic compensation at the Drosophila neuromuscular junction.Nat Commun. 2016 Jul 19;7:12188. doi: 10.1038/ncomms12188. Nat Commun. 2016. PMID: 27432119 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
