TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death
- PMID: 15775988
- PMCID: PMC556400
- DOI: 10.1038/sj.emboj.7600596
TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death
Abstract
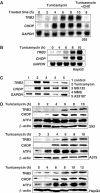
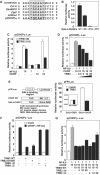
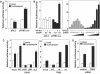
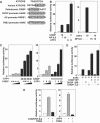
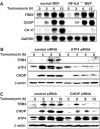
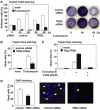
C/EBP homologous protein (CHOP) is a stress-inducible nuclear protein that is crucial for the development of programmed cell death and regeneration; however, the regulation of its function has not been well characterized. Slbo, a Drosophila homolog of C/EBP (CCAAT/enhancer binding protein), was shown to be unstabilized by tribbles. Here, we identified TRB3 as a tribbles ortholog in humans, which associated with CHOP to suppress the CHOP-dependent transactivation. TRB3 is induced by various forms endoplasmic reticulum (ER) stress later than CHOP. Tunicamycin treatment enhanced the TRB3 promoter activity, while dominant-negative forms of CHOP suppressed the tunicamycin-induced activation. In addition, the tunicamycin response region in the TRB3 promoter contains amino-acid response elements overlapping the CHOP-binding site, and CHOP and ATF4 cooperated to activate this promoter activity. Knockdown of endogenous ATF4 or CHOP expression dramatically repressed tunicamycin-induced TRB3 induction. Furthermore, knockdown of TRB3 expression decreased ER stress-dependent cell death. These results indicate that TRB3 is a novel target of CHOP/ATF4 and downregulates its own induction by repression of CHOP/ATF4 functions, and that it is involved in CHOP-dependent cell death during ER stress.
Figures


Similar articles
-
Tribble 3, a novel oxidized low-density lipoprotein-inducible gene, is induced via the activating transcription factor 4-C/EBP homologous protein pathway.Clin Exp Pharmacol Physiol. 2010 Jan;37(1):51-5. doi: 10.1111/j.1440-1681.2009.05229.x. Epub 2009 Jun 29. Clin Exp Pharmacol Physiol. 2010. PMID: 19566842
-
Characterization of human NIPK (TRB3, SKIP3) gene activation in stressful conditions.Biochem Biophys Res Commun. 2005 Apr 29;330(1):210-8. doi: 10.1016/j.bbrc.2005.02.149. Biochem Biophys Res Commun. 2005. PMID: 15781252
-
The molecular mechanism of endoplasmic reticulum stress-induced apoptosis in PC-12 neuronal cells: the protective effect of insulin-like growth factor I.Endocrinology. 2009 Jan;150(1):277-85. doi: 10.1210/en.2008-0794. Epub 2008 Sep 18. Endocrinology. 2009. PMID: 18801901
-
Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls.Genes Dev. 1999 May 15;13(10):1211-33. doi: 10.1101/gad.13.10.1211. Genes Dev. 1999. PMID: 10346810 Review. No abstract available.
-
[Molecular biology of the ER stress response].Seikagaku. 2004 Jul;76(7):617-30. Seikagaku. 2004. PMID: 15346898 Review. Japanese. No abstract available.
Cited by
-
Effects of PGC-1α overexpression on the myogenic response during skeletal muscle regeneration.Sports Med Health Sci. 2022 Jul 20;4(3):198-208. doi: 10.1016/j.smhs.2022.06.005. eCollection 2022 Sep. Sports Med Health Sci. 2022. PMID: 36090923 Free PMC article.
-
Specific Compositions of Cannabis sativa Compounds Have Cytotoxic Activity and Inhibit Motility and Colony Formation of Human Glioblastoma Cells In Vitro.Cancers (Basel). 2021 Apr 5;13(7):1720. doi: 10.3390/cancers13071720. Cancers (Basel). 2021. PMID: 33916466 Free PMC article.
-
ATF4 in cellular stress, ferroptosis, and cancer.Arch Toxicol. 2024 Apr;98(4):1025-1041. doi: 10.1007/s00204-024-03681-x. Epub 2024 Feb 21. Arch Toxicol. 2024. PMID: 38383612 Review.
-
Enhancing terminal erythroid differentiation in human embryonic stem cells through TRIB3 overexpression.Heliyon. 2024 Sep 5;10(18):e37463. doi: 10.1016/j.heliyon.2024.e37463. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39309892 Free PMC article.
-
Endoplasmic Reticulum Stress and Unfolded Protein Response Pathways: Potential for Treating Age-related Retinal Degeneration.J Ophthalmic Vis Res. 2012 Jan;7(1):45-59. J Ophthalmic Vis Res. 2012. PMID: 22737387 Free PMC article.
References
-
- Averous J, Bruhat A, Carraro V, Thiel G, Fafournoux P (2003) Induction of CHOP expression by amino acid limitation requires both ATF4 expression and ATF2 phosphorylation. J Biol Chem 279: 5288–5297 - PubMed
-
- Barone MV, Crozat A, Tabaee A, Philipson L, Ron D (1994) CHOP (GADD153) and its oncogenic variant, TLS-CHOP, have opposing effects on the induction of G1/S arrest. Genes Dev 8: 453–464 - PubMed
-
- Bowers AJ, Scully S, Boylan JF (2003) SKIP3, a novel Drosophila tribbles ortholog, is overexpressed in human tumors and is regulated by hypoxia. Oncogene 22: 2823–2835 - PubMed
-
- Brazil DP, Hemmings BA (2001) Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci 26: 657–664 - PubMed
-
- Bruhat A, Averous J, Carraro V, Zhong C, Reimold AM, Kilberg MS, Fafournoux P (2002) Differences in the molecular mechanisms involved in the transcriptional activation of the CHOP and Asparagine synthetase genes in response to amino acid deprivation or activation of the unfolded protein response. J Biol Chem 277: 48107–48114 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

