Stimulus-dependent changes in spike threshold enhance feature selectivity in rat barrel cortex neurons
- PMID: 15772358
- PMCID: PMC6725135
- DOI: 10.1523/JNEUROSCI.4906-04.2005
Stimulus-dependent changes in spike threshold enhance feature selectivity in rat barrel cortex neurons
Abstract
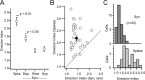
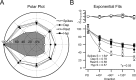
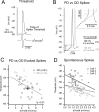
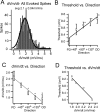
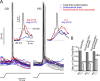
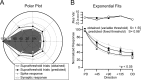
Feature selectivity is a fundamental property of sensory cortex neurons, yet the mechanisms underlying its genesis are not fully understood. Using intracellular recordings in vivo from layers 2-6 of rat barrel cortex, we studied the selectivity of neurons to the angular direction of whisker deflection. The spike output and the underlying synaptic response decreased exponentially in magnitude as the direction of deflection diverged from the preferred. However, the spike output was more sharply tuned for direction than the underlying synaptic response amplitude. This difference in selectivity was attributable to the rectification imposed by the spike threshold on the input-output function of cells. As in the visual system, spike threshold was not constant and showed trial-to-trial variability. However, here we show that the mean spike threshold was direction dependent and increased as the direction diverged from the preferred. Spike threshold was also related to the rate of rise of the synaptic response, which was direction dependent and steepest for the preferred direction. To assess the impact of the direction-dependent changes in spike threshold on direction selectivity, we applied a fixed threshold to the synaptic responses and calculated a predicted spike output. The predicted output was more broadly tuned than the obtained spike response, demonstrating for the first time that the regulation of the spike threshold by the properties of the synaptic response effectively enhances the selectivity of the spike output.
Figures

Similar articles
-
Dynamics of excitation and inhibition underlying stimulus selectivity in rat somatosensory cortex.Nat Neurosci. 2005 Oct;8(10):1364-70. doi: 10.1038/nn1545. Epub 2005 Sep 11. Nat Neurosci. 2005. PMID: 16158064
-
Similarity of direction tuning among responses to stimulation of different whiskers in neurons of rat barrel cortex.J Neurophysiol. 2005 Sep;94(3):2004-18. doi: 10.1152/jn.00113.2004. Epub 2005 Jun 22. J Neurophysiol. 2005. PMID: 15972836
-
Angular tuning and velocity sensitivity in different neuron classes within layer 4 of rat barrel cortex.J Neurophysiol. 2004 Jan;91(1):223-9. doi: 10.1152/jn.00541.2003. Epub 2003 Sep 24. J Neurophysiol. 2004. PMID: 14507984
-
Spike timing and synaptic dynamics at the awake thalamocortical synapse.Prog Brain Res. 2005;149:91-105. doi: 10.1016/S0079-6123(05)49008-1. Prog Brain Res. 2005. PMID: 16226579 Review.
-
Population coding in somatosensory cortex.Curr Opin Neurobiol. 2002 Aug;12(4):441-7. doi: 10.1016/s0959-4388(02)00338-0. Curr Opin Neurobiol. 2002. PMID: 12139993 Review.
Cited by
-
Biophysical mechanism of spike threshold dependence on the rate of rise of the membrane potential by sodium channel inactivation or subthreshold axonal potassium current.J Comput Neurosci. 2013 Aug;35(1):1-17. doi: 10.1007/s10827-012-0436-2. Epub 2013 Jan 24. J Comput Neurosci. 2013. PMID: 23344915 Free PMC article.
-
Slope-based stochastic resonance: how noise enables phasic neurons to encode slow signals.PLoS Comput Biol. 2010 Jun 24;6(6):e1000825. doi: 10.1371/journal.pcbi.1000825. PLoS Comput Biol. 2010. PMID: 20585612 Free PMC article.
-
Phasic and tonic patterns of locus coeruleus output differentially modulate sensory network function in the awake rat.J Neurophysiol. 2011 Jan;105(1):69-87. doi: 10.1152/jn.00445.2010. Epub 2010 Oct 27. J Neurophysiol. 2011. PMID: 20980542 Free PMC article.
-
Impact of fast sodium channel inactivation on spike threshold dynamics and synaptic integration.PLoS Comput Biol. 2011 May;7(5):e1001129. doi: 10.1371/journal.pcbi.1001129. Epub 2011 May 5. PLoS Comput Biol. 2011. PMID: 21573200 Free PMC article.
-
Cortical Microcircuit Mechanisms of Mismatch Negativity and Its Underlying Subcomponents.Front Neural Circuits. 2020 Mar 31;14:13. doi: 10.3389/fncir.2020.00013. eCollection 2020. Front Neural Circuits. 2020. PMID: 32296311 Free PMC article. Review.
References
-
- Alonso JM, Usrey WM, Reid RC (1996) Precisely correlated firing in cells of the lateral geniculate nucleus. Nature 383: 815-819. - PubMed
-
- Azouz R, Gray CM (2003) Adaptive coincidence detection and dynamic gain control in visual cortical neurons in vivo. Neuron 37: 513-523. - PubMed
-
- Bonds AB (1989) Role of inhibition in the specification of orientation selectivity of cells in the cat striate cortex. Vis Neurosci 2: 41-55. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
