Epstein-Barr virus transforming protein LMP1 plays a critical role in virus production
- PMID: 15767441
- PMCID: PMC1061545
- DOI: 10.1128/JVI.79.7.4415-4424.2005
Epstein-Barr virus transforming protein LMP1 plays a critical role in virus production
Abstract
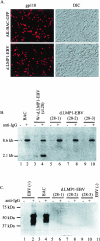
The Epstein-Barr virus (EBV) latent membrane protein 1 (LMP1), which is critical for EBV-induced B-cell transformation, is also abundantly expressed during the lytic cycle of viral replication. However, the biological significance of this strong LMP1 induction remains unknown. We engineered a bacterial artificial chromosome clone containing the entire genome of Akata strain EBV to specifically disrupt the LMP1 gene. Akata cell clones harboring the episomes of LMP1-deleted EBV were established, and the effect of LMP1 loss on virus production was investigated. We found that the degree of viral DNA amplification and the expression levels of viral late gene products were unaffected by LMP1 loss, demonstrating that the LMP1-deleted EBV entered the lytic replication cycle as efficiently as the wild-type counterpart. This was confirmed by our electron microscopic observation that nucleocapsid formation inside nuclei occurred even in the absence of LMP1. By contrast, loss of LMP1 severely impaired virus release into culture supernatants, resulting in poor infection efficiency. The expression of truncated LMP1 in Akata cells harboring LMP1-deleted EBV rescued the virus release into the culture supernatant and the infectivity, and full-length LMP1 partially rescued the infectivity. These results indicate that inducible expression of LMP1 during the viral lytic cycle plays a critical role in virus production.
Figures




Similar articles
-
Functional analysis of the mutated Epstein-Barr virus oncoprotein LMP1(69del): implications for a new role of naturally occurring LMP1 variants.Haematologica. 2003 Dec;88(12):1324-35. Haematologica. 2003. PMID: 14687985
-
Latent membrane protein 1 is critical for efficient growth transformation of human B cells by epstein-barr virus.Cancer Res. 2003 Jun 1;63(11):2982-9. Cancer Res. 2003. PMID: 12782607
-
Epstein-Barr virus infection in precursor lesions of nasopharyngeal carcinoma.Ai Zheng. 2006 Feb;25(2):136-42. Ai Zheng. 2006. PMID: 16480574
-
Regulation and dysregulation of Epstein-Barr virus latency: implications for the development of autoimmune diseases.Autoimmunity. 2008 May;41(4):298-328. doi: 10.1080/08916930802024772. Autoimmunity. 2008. PMID: 18432410 Review.
-
Role of Epstein-Barr virus encoded latent membrane protein 1 in the carcinogenesis of nasopharyngeal carcinoma.Cell Mol Immunol. 2007 Jun;4(3):185-96. Cell Mol Immunol. 2007. PMID: 17601372 Review.
Cited by
-
Functions of the Epstein-Barr virus EBNA1 protein in viral reactivation and lytic infection.J Virol. 2012 Jun;86(11):6146-58. doi: 10.1128/JVI.00013-12. Epub 2012 Apr 4. J Virol. 2012. PMID: 22491455 Free PMC article.
-
Herpesvirus BACs: past, present, and future.J Biomed Biotechnol. 2011;2011:124595. doi: 10.1155/2011/124595. Epub 2010 Oct 27. J Biomed Biotechnol. 2011. PMID: 21048927 Free PMC article. Review.
-
Therapeutic evaluation of Epstein-Barr virus-encoded latent membrane protein-1 targeted DNAzyme for treating of nasopharyngeal carcinomas.Mol Ther. 2014 Feb;22(2):371-377. doi: 10.1038/mt.2013.257. Epub 2013 Oct 28. Mol Ther. 2014. PMID: 24322331 Free PMC article. Clinical Trial.
-
Epstein-Barr virus perpetuates B cell germinal center dynamics and generation of autoimmune-associated phenotypes in vitro.Front Immunol. 2022 Sep 28;13:1001145. doi: 10.3389/fimmu.2022.1001145. eCollection 2022. Front Immunol. 2022. PMID: 36248899 Free PMC article.
-
Virus and cell RNAs expressed during Epstein-Barr virus replication.J Virol. 2006 Mar;80(5):2548-65. doi: 10.1128/JVI.80.5.2548-2565.2006. J Virol. 2006. PMID: 16474161 Free PMC article.
References
-
- Baer, R., A. T. Bankier, M. D. Biggin, P. L. Deininger, P. J. Farrell, T. J. Gibson, G. Hatfull, G. S. Hudson, S. C. Satchwell, C. Seguin, et al. 1984. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature 310:207-211. - PubMed
-
- Baichwal, V. R., and B. Sugden. 1989. The multiple membrane-spanning segments of the BNLF-1 oncogene from Epstein-Barr virus are required for transformation. Oncogene 4:67-74. - PubMed
-
- Boos, H., R. Berger, C. Kuklik-Roos, T. Iftner, and N. Mueller-Lantzsch. 1987. Enhancement of Epstein-Barr virus membrane protein (LMP) expression by serum, TPA, or n-butyrate in latently infected Raji cells. Virology 159:161-165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

