Expression of glucose transporter 1 confers susceptibility to human T-cell leukemia virus envelope-mediated fusion
- PMID: 15767416
- PMCID: PMC1061550
- DOI: 10.1128/JVI.79.7.4150-4158.2005
Expression of glucose transporter 1 confers susceptibility to human T-cell leukemia virus envelope-mediated fusion
Abstract
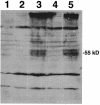
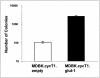
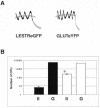
Human T-cell leukemia virus type 1 (HTLV-1) was the first human retrovirus identified and causes both adult T-cell leukemia/lymphoma and tropical spastic paraparesis/HTLV-1-associated myelopathy, among other disorders. In vitro, HTLV-1 has an extremely broad host cell tropism in that it is capable of infecting most mammalian cell types, although at the same time viral titers remain relatively low. Despite years of study, only recently has a bona fide candidate cellular receptor, glucose transporter 1 (glut-1), been identified. Although glut-1 was shown to bind specifically to the ectodomain of HTLV-1 and HTLV-2 envelope glycoproteins, which was reversible with small interfering RNA directed against glut-1, cellular susceptibility to HTLV upon expression of glut-1 was not established. Here we show that expression of glut-1 in relatively resistant MDBK cells conferred increased susceptibility to both HTLV-1- and HTLV-2-pseudotyped particles. glut-1 also markedly increased syncytium formation in MDBK cells after exposure to HTLV-1. Another assay also demonstrated HTLV-1 envelope-cell fusion in the presence of glut-1. Taken together, these results provide additional evidence that glut-1 is a receptor for HTLV.
Figures




Similar articles
-
Isolated receptor binding domains of HTLV-1 and HTLV-2 envelopes bind Glut-1 on activated CD4+ and CD8+ T cells.Retrovirology. 2007 May 15;4:31. doi: 10.1186/1742-4690-4-31. Retrovirology. 2007. PMID: 17504522 Free PMC article.
-
The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV.Cell. 2003 Nov 14;115(4):449-59. doi: 10.1016/s0092-8674(03)00881-x. Cell. 2003. PMID: 14622599
-
Infection of CD4+ T lymphocytes by the human T cell leukemia virus type 1 is mediated by the glucose transporter GLUT-1: evidence using antibodies specific to the receptor's large extracellular domain.Virology. 2006 May 25;349(1):184-96. doi: 10.1016/j.virol.2006.01.045. Epub 2006 Mar 7. Virology. 2006. PMID: 16519917
-
HTLV-1 tropism and envelope receptor.Oncogene. 2005 Sep 5;24(39):6016-25. doi: 10.1038/sj.onc.1208972. Oncogene. 2005. PMID: 16155608 Review.
-
Tropical spastic paraparesis and HTLV-1 associated myelopathy: clinical, epidemiological, virological and therapeutic aspects.Rev Neurol (Paris). 2012 Mar;168(3):257-69. doi: 10.1016/j.neurol.2011.12.006. Epub 2012 Mar 7. Rev Neurol (Paris). 2012. PMID: 22405461 Review.
Cited by
-
Isolated receptor binding domains of HTLV-1 and HTLV-2 envelopes bind Glut-1 on activated CD4+ and CD8+ T cells.Retrovirology. 2007 May 15;4:31. doi: 10.1186/1742-4690-4-31. Retrovirology. 2007. PMID: 17504522 Free PMC article.
-
Entry of human T-cell leukemia virus type 1 is augmented by heparin sulfate proteoglycans bearing short heparin-like structures.J Virol. 2012 Mar;86(6):2959-69. doi: 10.1128/JVI.05783-11. Epub 2012 Jan 11. J Virol. 2012. PMID: 22238310 Free PMC article.
-
Isolation and characterization of mouse-human microcell hybrid cell clones permissive for infectious HIV particle release.Virology. 2007 Jun 5;362(2):283-93. doi: 10.1016/j.virol.2006.12.015. Epub 2007 Jan 31. Virology. 2007. PMID: 17270231 Free PMC article.
-
Human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 use different receptor complexes to enter T cells.J Virol. 2006 Sep;80(17):8291-302. doi: 10.1128/JVI.00389-06. J Virol. 2006. PMID: 16912281 Free PMC article.
-
Recent Updates on Viral Oncogenesis: Available Preventive and Therapeutic Entities.Mol Pharm. 2023 Aug 7;20(8):3698-3740. doi: 10.1021/acs.molpharmaceut.2c01080. Epub 2023 Jul 24. Mol Pharm. 2023. PMID: 37486263 Free PMC article. Review.
References
-
- Blattner, W. A., D. W. Blayney, G. M. Robert, M. G. Sarngadharan, V. S. Kalyanaraman, P. S. Sarin, E. S. Jaffe, and R. C. Gallo. 1983. Epidemiology of human T-cell leukemia/lymphoma virus. J. Infect. Dis. 147:406-416. - PubMed
-
- Blattner, W. A., V. S. Kalyanaraman, G. M. Robert, T. A. Lister, D. A. Galton, P. S. Sarin, M. H. Crawford, D. Catovsky, M. Greaves, and R. C. Gallo. 1982. The human type-C retrovirus, HTLV, in Blacks from the Caribbean region, and relationship to adult T-cell leukemia/lymphoma. Int. J. Cancer 30:257-264. - PubMed
-
- Blayney, D. W., W. A. Blattner, G. M. Robert, E. S. Jaffe, R. I. Fisher, P. J. Bunn, M. G. Patton, H. R. Rarick, and R. C. Gallo. 1983. The human T-cell leukemia-lymphoma virus in the southeastern United States. JAMA 250:1048-1052. - PubMed
-
- Bolz, S., C. L. Farrell, K. Dietz, and H. Wolburg. 1996. Subcellular distribution of glucose transporter (GLUT-1) during development of the blood-brain barrier in rats. Cell Tissue Res. 284:355-365. - PubMed
-
- Clapham, P., K. Nagy, P. R. Cheingsong, M. Exley, and R. A. Weiss. 1983. Productive infection and cell-free transmission of human T-cell leukemia virus in a nonlymphoid cell line. Science 222:1125-1127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

