Retrovirus restriction by TRIM5alpha variants from Old World and New World primates
- PMID: 15767395
- PMCID: PMC1061569
- DOI: 10.1128/JVI.79.7.3930-3937.2005
Retrovirus restriction by TRIM5alpha variants from Old World and New World primates
Abstract
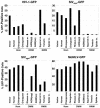
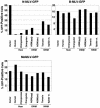
The TRIM5alpha proteins of humans and some Old World monkeys have been shown to block infection of particular retroviruses following virus entry into the host cell. Infection of most New World monkey cells by the simian immunodeficiency virus of macaques (SIVmac) is restricted at a similar point. Here we examine the antiretroviral activity of TRIM5alpha orthologs from humans, apes, Old World monkeys, and New World monkeys. Chimpanzee and orangutan TRIM5alpha proteins functionally resembled human TRIM5alpha, potently restricting infection by N-tropic murine leukemia virus (N-MLV) and moderately restricting human immunodeficiency virus type 1 (HIV-1) infection. Notably, TRIM5alpha proteins from several New World monkey species restricted infection by SIVmac and the SIV of African green monkeys, SIVagm. Spider monkey TRIM5alpha, which has an expanded B30.2 domain v3 region due to a tandem triplication, potently blocked infection by a range of retroviruses, including SIVmac, SIVagm, HIV-1, and N-MLV. Tandem duplications in the TRIM5alpha B30.2 domain v1 region of African green monkeys are also associated with broader antiretroviral activity. Thus, variation in TRIM5alpha proteins among primate species accounts for the observed patterns of postentry restrictions in cells from these animals. The TRIM5alpha proteins of some monkey species exhibit dramatic lengthening of particular B30.2 variable regions and an expanded range of susceptible retroviruses.
Figures



Similar articles
-
Species-specific variation in the B30.2(SPRY) domain of TRIM5alpha determines the potency of human immunodeficiency virus restriction.J Virol. 2005 Mar;79(5):3139-45. doi: 10.1128/JVI.79.5.3139-3145.2005. J Virol. 2005. PMID: 15709033 Free PMC article.
-
A specific region of 37 amino acid residues in the SPRY (B30.2) domain of African green monkey TRIM5alpha determines species-specific restriction of simian immunodeficiency virus SIVmac infection.J Virol. 2005 Jul;79(14):8870-7. doi: 10.1128/JVI.79.14.8870-8877.2005. J Virol. 2005. PMID: 15994780 Free PMC article.
-
Isolation of an active Lv1 gene from cattle indicates that tripartite motif protein-mediated innate immunity to retroviral infection is widespread among mammals.J Virol. 2006 Aug;80(15):7332-8. doi: 10.1128/JVI.00516-06. J Virol. 2006. PMID: 16840314 Free PMC article.
-
TRIM5alpha.Curr Top Microbiol Immunol. 2009;339:47-66. doi: 10.1007/978-3-642-02175-6_3. Curr Top Microbiol Immunol. 2009. PMID: 20012523 Review.
-
[TRIM5alpha].Uirusu. 2005 Dec;55(2):259-65. doi: 10.2222/jsv.55.259. Uirusu. 2005. PMID: 16557011 Review. Japanese.
Cited by
-
Birth, decay, and reconstruction of an ancient TRIMCyp gene fusion in primate genomes.Proc Natl Acad Sci U S A. 2013 Feb 12;110(7):E583-92. doi: 10.1073/pnas.1216542110. Epub 2013 Jan 14. Proc Natl Acad Sci U S A. 2013. PMID: 23319649 Free PMC article.
-
Primate TRIM5 proteins form hexagonal nets on HIV-1 capsids.Elife. 2016 Jun 2;5:e16269. doi: 10.7554/eLife.16269. Elife. 2016. PMID: 27253068 Free PMC article.
-
Antiviral Activity of Feline BCA2 Is Mainly Dependent on Its Interference With Proviral Transcription Rather Than Degradation of FIV Gag.Front Microbiol. 2020 Jun 11;11:1230. doi: 10.3389/fmicb.2020.01230. eCollection 2020. Front Microbiol. 2020. PMID: 32595622 Free PMC article.
-
Unique Mode of Antiviral Action of a Marine Alkaloid against Ebola Virus and SARS-CoV-2.Viruses. 2022 Apr 15;14(4):816. doi: 10.3390/v14040816. Viruses. 2022. PMID: 35458549 Free PMC article.
-
A phenotypic recessive, post-entry block in rabbit cells that results in aberrant trafficking of HIV-1.Traffic. 2006 Aug;7(8):978-92. doi: 10.1111/j.1600-0854.2006.00449.x. Traffic. 2006. PMID: 16882040 Free PMC article.
References
-
- Artes, E. J., and M. A. Wainberg. 1996. Human immunodeficiency type 1 reverse transcriptase and early events in reverse transcription. Adv. Virus Res. 46:97-163. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

