Regulation of anaphylactic responses by phosphatidylinositol phosphate kinase type I {alpha}
- PMID: 15767368
- PMCID: PMC2213097
- DOI: 10.1084/jem.20041891
Regulation of anaphylactic responses by phosphatidylinositol phosphate kinase type I {alpha}
Abstract
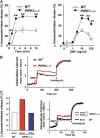
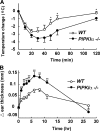
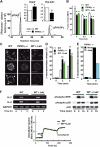
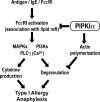
The membrane phospholipid phosphatidylinositol 4, 5-bisphosphate [PI(4,5)P(2)] is a critical signal transducer in eukaryotic cells. However, the physiological roles of the type I phosphatidylinositol phosphate kinases (PIPKIs) that synthesize PI(4,5)P(2) are largely unknown. Here, we show that the alpha isozyme of PIPKI (PIPKIalpha) negatively regulates mast cell functions and anaphylactic responses. In vitro, PIPKIalpha-deficient mast cells exhibited increased degranulation and cytokine production after Fcepsilon receptor-I cross-linking. In vivo, PIPKIalpha(-/-) mice displayed enhanced passive cutaneous and systemic anaphylaxis. Filamentous actin was diminished in PIPKIalpha(-/-) mast cells, and enhanced degranulation observed in the absence of PIPKIalpha was also seen in wild-type mast cells treated with latrunculin, a pharmacological inhibitor of actin polymerization. Moreover, the association of FcepsilonRI with lipid rafts and FcepsilonRI-mediated activation of signaling proteins was augmented in PIPKIalpha(-/-) mast cells. Thus, PIPKIalpha is a negative regulator of FcepsilonRI-mediated cellular responses and anaphylaxis, which functions by controlling the actin cytoskeleton and dynamics of FcepsilonRI signaling. Our results indicate that the different PIPKI isoforms might be functionally specialized.
Figures
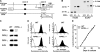


Similar articles
-
[Compartmentalization of phosphatidylinositol 4, 5-bisphosphate].Rinsho Byori. 2006 Jan;54(1):45-50. Rinsho Byori. 2006. PMID: 16499229 Review. Japanese.
-
Tetraspanin CD151 Is a Negative Regulator of FcεRI-Mediated Mast Cell Activation.J Immunol. 2015 Aug 15;195(4):1377-87. doi: 10.4049/jimmunol.1302874. Epub 2015 Jul 1. J Immunol. 2015. PMID: 26136426 Free PMC article.
-
Sphingosine kinase 1 is pivotal for Fc epsilon RI-mediated mast cell signaling and functional responses in vitro and in vivo.J Immunol. 2009 Jul 1;183(1):221-7. doi: 10.4049/jimmunol.0803430. J Immunol. 2009. Retraction in: J Immunol. 2012 Sep 15;189(6):3262. doi: 10.4049/jimmunol.1290050 PMID: 19542433 Retracted.
-
SWAP-70 regulates mast cell FcepsilonRI-mediated signaling and anaphylaxis.Eur J Immunol. 2008 Mar;38(3):841-54. doi: 10.1002/eji.200737597. Eur J Immunol. 2008. PMID: 18236401 Free PMC article.
-
Phosphatidylinositol 4, 5 bisphosphate and the actin cytoskeleton.Subcell Biochem. 2012;59:177-215. doi: 10.1007/978-94-007-3015-1_6. Subcell Biochem. 2012. PMID: 22374091 Review.
Cited by
-
Phosphoinositides: tiny lipids with giant impact on cell regulation.Physiol Rev. 2013 Jul;93(3):1019-137. doi: 10.1152/physrev.00028.2012. Physiol Rev. 2013. PMID: 23899561 Free PMC article. Review.
-
Phosphatidylinositol-4-phosphate-5-kinase alpha deficiency alters dynamics of glucose-stimulated insulin release to improve glucohomeostasis and decrease obesity in mice.Diabetes. 2011 Feb;60(2):454-63. doi: 10.2337/db10-0614. Diabetes. 2011. PMID: 21270258 Free PMC article.
-
Regulation of HGF-induced hepatocyte proliferation by the small GTPase Arf6 through the PIP2-producing enzyme PIP5K1A.Sci Rep. 2017 Aug 25;7(1):9438. doi: 10.1038/s41598-017-09633-z. Sci Rep. 2017. PMID: 28842595 Free PMC article.
-
Regulation of the actin cytoskeleton by phosphatidylinositol 4-phosphate 5 kinases.Pflugers Arch. 2007 Oct;455(1):5-18. doi: 10.1007/s00424-007-0286-3. Epub 2007 May 23. Pflugers Arch. 2007. PMID: 17520274 Review.
-
Phosphatidylinositol 4-phosphate 5-kinase alpha is induced in ganglioside-stimulated brain astrocytes and contributes to inflammatory responses.Exp Mol Med. 2010 Sep 30;42(9):662-73. doi: 10.3858/emm.2010.42.9.066. Exp Mol Med. 2010. PMID: 20720456 Free PMC article.
References
-
- Metcalfe, D.D., D. Baram, and Y.A. Mekori. 1997. Mast cells. Physiol. Rev. 77:1033–1079. - PubMed
-
- Kinet, J.P. 1999. The high-affinity IgE receptor (FcɛRI): from physiology to pathology. Annu. Rev. Immunol. 17:931–972. - PubMed
-
- Galli, S.J. 2000. Mast cells and basophils. Curr. Opin. Hematol. 7:32–39. - PubMed
-
- Turner, H., and J.P. Kinet. 1999. Signalling through the high-affinity IgE receptor FcɛRI. Nature. 402:B24–B30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

