Signaling by target of rapamycin proteins in cell growth control
- PMID: 15755954
- PMCID: PMC1082789
- DOI: 10.1128/MMBR.69.1.79-100.2005
Signaling by target of rapamycin proteins in cell growth control
Abstract
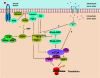
Target of rapamycin (TOR) proteins are members of the phosphatidylinositol kinase-related kinase (PIKK) family and are highly conserved from yeast to mammals. TOR proteins integrate signals from growth factors, nutrients, stress, and cellular energy levels to control cell growth. The ribosomal S6 kinase 1 (S6K) and eukaryotic initiation factor 4E binding protein 1(4EBP1) are two cellular targets of TOR kinase activity and are known to mediate TOR function in translational control in mammalian cells. However, the precise molecular mechanism of TOR regulation is not completely understood. One of the recent breakthrough studies in TOR signaling resulted in the identification of the tuberous sclerosis complex gene products, TSC1 and TSC2, as negative regulators for TOR signaling. Furthermore, the discovery that the small GTPase Rheb is a direct downstream target of TSC1-TSC2 and a positive regulator of the TOR function has significantly advanced our understanding of the molecular mechanism of TOR activation. Here we review the current understanding of the regulation of TOR signaling and discuss its function as a signaling nexus to control cell growth during normal development and tumorigenesis.
Figures
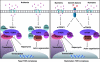
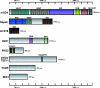
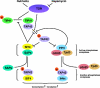
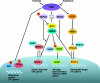

Similar articles
-
Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling.Genes Dev. 2003 Aug 1;17(15):1829-34. doi: 10.1101/gad.1110003. Epub 2003 Jul 17. Genes Dev. 2003. PMID: 12869586 Free PMC article.
-
Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb.Curr Biol. 2003 Aug 5;13(15):1259-68. doi: 10.1016/s0960-9822(03)00506-2. Curr Biol. 2003. PMID: 12906785
-
Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase.Oncogene. 2006 Oct 16;25(48):6361-72. doi: 10.1038/sj.onc.1209882. Oncogene. 2006. PMID: 17041622 Review.
-
Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression.Oncogene. 2004 Apr 19;23(18):3151-71. doi: 10.1038/sj.onc.1207542. Oncogene. 2004. PMID: 15094765 Review.
-
The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses.J Biol Chem. 2005 May 13;280(19):18717-27. doi: 10.1074/jbc.M414499200. Epub 2005 Mar 16. J Biol Chem. 2005. PMID: 15772076
Cited by
-
Excessive Leucine-mTORC1-Signalling of Cow Milk-Based Infant Formula: The Missing Link to Understand Early Childhood Obesity.J Obes. 2012;2012:197653. doi: 10.1155/2012/197653. Epub 2012 Mar 19. J Obes. 2012. PMID: 22523661 Free PMC article.
-
Rapamycin and mTOR: a serendipitous discovery and implications for breast cancer.Clin Transl Med. 2012 Nov 15;1(1):29. doi: 10.1186/2001-1326-1-29. Clin Transl Med. 2012. PMID: 23369283 Free PMC article.
-
Cyclin alterations in diverse cancers: Outcome and co-amplification network.Oncotarget. 2015 Feb 20;6(5):3033-42. doi: 10.18632/oncotarget.2848. Oncotarget. 2015. PMID: 25596748 Free PMC article.
-
Ammonia-specific regulation of Gln3 localization in Saccharomyces cerevisiae by protein kinase Npr1.J Biol Chem. 2006 Sep 22;281(38):28460-9. doi: 10.1074/jbc.M604171200. Epub 2006 Jul 24. J Biol Chem. 2006. PMID: 16864577 Free PMC article.
-
Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony.J Biol Chem. 2010 May 7;285(19):14071-7. doi: 10.1074/jbc.R109.094003. Epub 2010 Mar 15. J Biol Chem. 2010. PMID: 20231296 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

