Cytoprotective effects of geranylgeranylacetone against retinal photooxidative damage
- PMID: 15745966
- PMCID: PMC6726103
- DOI: 10.1523/JNEUROSCI.4866-04.2005
Cytoprotective effects of geranylgeranylacetone against retinal photooxidative damage
Abstract
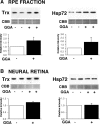
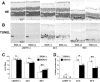
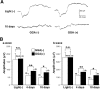
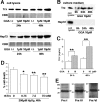
Exposure to excessive light induces retinal photoreceptor cell damage, leading to development and progression of various retinal diseases. We tested the effect of geranylgeranylacetone (GGA), an acyclic polyisoprenoid, on light-induced retinal damage in mice. Oral treatment with GGA (1.0 mg/d) for 5 d induced thioredoxin (Trx) and heat shock protein 72 (Hsp72) predominantly in the retinal pigment epithelium (RPE). After white light exposure (8000 lux for 2 h), the percentage of terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling-positive photoreceptor cells decreased significantly at 24 and 96 h, and the number of photoreceptor cell nuclei at 96 h and the electroretinographic amplitudes of the a- and b-waves at 4 and 10 d increased significantly in GGA-pretreated mice compared with saline-pretreated mice. Light-induced upregulations of 8-hydroxy-2-deoxyguanosine and 4-hydroxy-2-nonenal-modified protein, markers of oxidative stress, were inhibited by GGA pretreatment. To elucidate the cytoprotective mechanism of GGA and Trx, we used human K-1034 RPE cells and mouse photoreceptor-derived 661W cells. In K-1034 cells, GGA (10 microM) induced intracellular Trx, Hsp72, and extracellular Trx but not extracellular Hsp72. Extracellular Trx (0.75 nM) attenuated H2O2 (200 microM)-induced cell damage in 661W cells. Pretreatment with GGA and overexpression of Trx in K-1034 cells counteracted H2O2 (50 microM)-induced attenuation of cellular latex bead incorporation. Protection of phagocytotic activity through induction of Trx and possibly Hsp72 in RPE cells and elimination of oxidative stress in the photoreceptor layer through release of Trx from RPE cells may be mechanisms of GGA-mediated cytoprotection. Therefore, Trx is a neurotrophic factor released from RPE cells and plays a crucial role in maintaining photoreceptor cell integrity.
Figures


Similar articles
-
Sulforaphane induces thioredoxin through the antioxidant-responsive element and attenuates retinal light damage in mice.Invest Ophthalmol Vis Sci. 2005 Mar;46(3):979-87. doi: 10.1167/iovs.04-1120. Invest Ophthalmol Vis Sci. 2005. PMID: 15728556
-
Cytoprotective effect of thioredoxin against retinal photic injury in mice.Invest Ophthalmol Vis Sci. 2002 Apr;43(4):1162-7. Invest Ophthalmol Vis Sci. 2002. PMID: 11923261
-
Retinal ganglion cell protection with geranylgeranylacetone, a heat shock protein inducer, in a rat glaucoma model.Invest Ophthalmol Vis Sci. 2003 May;44(5):1982-92. Invest Ophthalmol Vis Sci. 2003. PMID: 12714633
-
Thioredoxin superfamily and thioredoxin-inducing agents.Ann N Y Acad Sci. 2002 May;957:189-99. doi: 10.1111/j.1749-6632.2002.tb02916.x. Ann N Y Acad Sci. 2002. PMID: 12074972 Review.
-
Morphine Addiction and Oxidative Stress: The Potential Effects of Thioredoxin-1.Front Pharmacol. 2020 Feb 21;11:82. doi: 10.3389/fphar.2020.00082. eCollection 2020. Front Pharmacol. 2020. PMID: 32153403 Free PMC article. Review.
Cited by
-
Overexpression of thioredoxins 1 and 2 increases retinal ganglion cell survival after pharmacologically induced oxidative stress, optic nerve transection, and in experimental glaucoma.Trans Am Ophthalmol Soc. 2009 Dec;107:161-5. Trans Am Ophthalmol Soc. 2009. PMID: 20126492 Free PMC article.
-
Suppression of Alzheimer's disease-related phenotypes by geranylgeranylacetone in mice.PLoS One. 2013 Oct 1;8(10):e76306. doi: 10.1371/journal.pone.0076306. eCollection 2013. PLoS One. 2013. PMID: 24098472 Free PMC article.
-
Growth factor receptor-bound protein 14 undergoes light-dependent intracellular translocation in rod photoreceptors: functional role in retinal insulin receptor activation.Biochemistry. 2009 Jun 23;48(24):5563-72. doi: 10.1021/bi9000062. Biochemistry. 2009. PMID: 19438210 Free PMC article.
-
Inhibition of ASK1-p38 pathway prevents neural cell death following optic nerve injury.Cell Death Differ. 2013 Feb;20(2):270-80. doi: 10.1038/cdd.2012.122. Epub 2012 Sep 14. Cell Death Differ. 2013. PMID: 22976835 Free PMC article.
-
Suppressing endoplasmic reticulum stress-related autophagy attenuates retinal light injury.Aging (Albany NY). 2020 Aug 28;12(16):16579-16596. doi: 10.18632/aging.103846. Epub 2020 Aug 28. Aging (Albany NY). 2020. PMID: 32858529 Free PMC article.
References
-
- Bai J, Nakamura H, Hattori I, Tanito M, Yo J (2002) Thioredoxin suppresses 1-methyl-4-phenylpyridinium-induced neurotoxicity in rat PC12 cells. Neurosci Lett 321: 81-84. - PubMed
-
- Bailey TA, Kanuga N, Romero IA, Greenwood J, Luthert PJ, Cheetham ME (2004) Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 45: 675-684. - PubMed
-
- Barbe MF, Tytell M, Gower DJ, Welch WJ (1988) Hyperthermia protects against light damage in the rat retina. Science 241: 1817-1820. - PubMed
-
- Bilski J, Murty VL, Nadziejko C, Sarosiek J, Aono M, Moriga M, Slomiany A, Slomiany BL (1988) Protection against alcohol-induced gastric mucosal injury by geranylgeranylacetone: effect of indomethacin. Digestion 41: 22-33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
