The multiple functions of cysteine-string protein analyzed at Drosophila nerve terminals
- PMID: 15745946
- PMCID: PMC6726096
- DOI: 10.1523/JNEUROSCI.3610-04.2005
The multiple functions of cysteine-string protein analyzed at Drosophila nerve terminals
Abstract
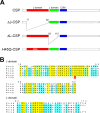
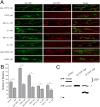
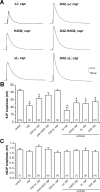
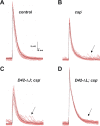
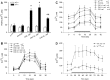
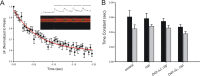
The synaptic vesicle-associated cysteine-string protein (CSP) is important for synaptic transmission. Previous studies revealed multiple defects at neuromuscular junctions (NMJs) of csp null-mutant Drosophila, but whether these defects are independent of each other or mechanistically linked through J domain mediated-interactions with heat-shock cognate protein 70 (Hsc70) has not been established. To resolve this issue, we genetically dissected the individual functions of CSP by an in vivo structure/function analysis. Expression of mutant CSP lacking the J domain at csp null-mutant NMJs fully restored normal thermo-tolerance of evoked transmitter release but did not completely restore evoked release at room temperature and failed to reverse the abnormal intraterminal Ca2+ levels. This suggests that J domain-mediated functions are essential for the regulation of intraterminal Ca2+ levels but only partially required for regulating evoked release and not required for protecting evoked release against thermal stress. Hence, CSP can also act as an Hsc70-independent chaperone protecting evoked release from thermal stress. Expression of mutant CSP lacking the L domain restored neurotransmission and partially reversed the abnormal intraterminal Ca2+ levels, suggesting that the L domain is important, although not essential, for the role of CSP in regulating intraterminal Ca2+ levels. We detected no effects of csp mutations on individual presynaptic Ca2+ signals triggered by action potentials, suggesting that presynaptic Ca2+ entry is not primarily impaired. Both the J and L domains were also required for the role of CSP in synaptic growth. Together, these results suggest that CSP has several independent synaptic functions, affecting synaptic growth, evoked release, thermal protection of evoked release, and intraterminal Ca2+ levels at rest and during stimulation.
Figures
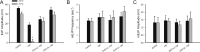
Similar articles
-
Morphological and functional effects of altered cysteine string protein at the Drosophila larval neuromuscular junction.Synapse. 2007 Jan;61(1):1-16. doi: 10.1002/syn.20335. Synapse. 2007. PMID: 17068777
-
Cysteine-string protein increases the calcium sensitivity of neurotransmitter exocytosis in Drosophila.J Neurosci. 2000 Aug 15;20(16):6039-47. doi: 10.1523/JNEUROSCI.20-16-06039.2000. J Neurosci. 2000. PMID: 10934253 Free PMC article.
-
Presynaptic calcium-channel currents in normal and csp mutant Drosophila peptidergic terminals.Eur J Neurosci. 1999 May;11(5):1818-26. doi: 10.1046/j.1460-9568.1999.00604.x. Eur J Neurosci. 1999. PMID: 10215934
-
Cysteine string proteins.Prog Neurobiol. 2020 May;188:101758. doi: 10.1016/j.pneurobio.2020.101758. Epub 2020 Feb 7. Prog Neurobiol. 2020. PMID: 32044380 Review.
-
Cysteine-string protein: the chaperone at the synapse.J Neurochem. 2000 May;74(5):1781-9. doi: 10.1046/j.1471-4159.2000.0741781.x. J Neurochem. 2000. PMID: 10800920 Review.
Cited by
-
Depression in the fly.J Neurosci. 2006 Aug 2;26(31):8021-2. doi: 10.1523/jneurosci.2497-06.2006. J Neurosci. 2006. PMID: 16888835 Free PMC article. No abstract available.
-
Phosphorylation of Cysteine String Protein Triggers a Major Conformational Switch.Structure. 2016 Aug 2;24(8):1380-1386. doi: 10.1016/j.str.2016.06.009. Epub 2016 Jul 21. Structure. 2016. PMID: 27452402 Free PMC article.
-
Heat shock response and homeostatic plasticity.Front Cell Neurosci. 2015 Mar 12;9:68. doi: 10.3389/fncel.2015.00068. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25814928 Free PMC article.
-
MARCKS regulation of mucin secretion by airway epithelium in vitro: interaction with chaperones.Am J Respir Cell Mol Biol. 2008 Jul;39(1):68-76. doi: 10.1165/rcmb.2007-0139OC. Epub 2008 Feb 28. Am J Respir Cell Mol Biol. 2008. PMID: 18314541 Free PMC article.
-
CSPα-chaperoning presynaptic proteins.Front Cell Neurosci. 2014 Apr 29;8:116. doi: 10.3389/fncel.2014.00116. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24808827 Free PMC article. Review.
References
-
- Arnold C, Reisch N, Leibold C, Becker S, Prufert K, Sautter K, Palm D, Jatzke S, Buchner S, Buchner E (2004) Structure-function analysis of the cysteine string protein in Drosophila: cysteine string, linker and C terminus. J Exp Biol 207: 1323-1334. - PubMed
-
- Barclay JW, Atwood HL, Robertson RM (2002) Impairment of central pattern generation in Drosophila cysteine string protein mutants. J Comp Physiol 188: 71-78. - PubMed
-
- Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401-415. - PubMed
-
- Braun J, Wilbanks SM, Scheller RH (1996) The cysteine string secretory vesicle protein activates Hsc70 ATPase. J Biol Chem 271: 25989-25993. - PubMed
-
- Bronk P, Wenniger JJ, Dawson-Scully K, Guo X, Hong S, Atwood HL, Zinsmaier KE (2001) Drosophila Hsc70-4 is critical for neurotransmitter exocytosis in vivo. Neuron 30: 475-488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

