High-density lipoprotein as a potential carrier for delivery of a lipophilic antitumoral drug into hepatoma cells
- PMID: 15742395
- PMCID: PMC4250784
- DOI: 10.3748/wjg.v11.i7.954
High-density lipoprotein as a potential carrier for delivery of a lipophilic antitumoral drug into hepatoma cells
Abstract
Aim: To investigate the possibility of recombinant high-density lipoprotein (rHDL) being a carrier for delivering antitumoral drug to hepatoma cells.
Methods: Recombinant complex of HDL and aclacinomycin (rHDL-ACM) was prepared by cosonication of apoproteins from HDL (Apo HDL) and ACM as well as phosphatidylcholine. Characteristics of the rHDL-ACM were elucidated by electrophoretic mobility, including the size of particles, morphology and entrapment efficiency. Binding activity of rHDL-ACM to human hepatoma cells was determined by competition assay in the presence of excess native HDL. The cytotoxicity of rHDL-ACM was assessed by MTT method.
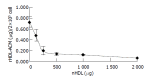
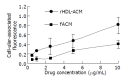
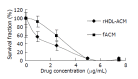
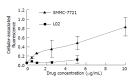
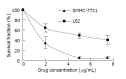
Results: The density range of rHDL-ACM was 1.063-1.210 g/mL, and the same as that of native HDL. The purity of all rHDL-ACM preparations was more than 92%. Encapsulated efficiencies of rHDL-ACM were more than 90%. rHDL-ACM particles were typical sphere model of lipoproteins and heterogeneous in particle size. The average diameter was 31.26+/-5.62 nm by measure of 110 rHDL-ACM particles in the range of diameter of lipoproteins. rHDL-ACM could bind on SMMC-7721 cells, and such binding could be competed against in the presence of excess native HDL. rHDL-ACM had same binding capacity as native HDL. The cellular uptake of rHDL-ACM by SMMC-7721 hepatoma cells was significantly higher than that of free ACM at the concentration range of 0.5-10 microg/mL (P<0.01). Cytotoxicity of rHDL-ACM to SMMC-7721 cells was significantly higher than that of free ACM at concentration range of less than 5 microg/mL (P<0.01) and IC50 of rHDL-ACM was lower than IC50 of free ACM (1.68 nmol/L vs 3 nmol/L). Compared to L02 hepatocytes, a normal liver cell line, the cellular uptake of rHDL-ACM by SMMC-7721 cells was significantly higher (P<0.01) and in a dose-dependent manner at the concentration range of 0.5-10 microg/mL. Cytotoxicity of the rHDL-ACM to SMMC-7721 cells was significantly higher than that to L02 cells at concentration range of 1-7.5 microg/mL (P<0.01). IC50 for SMMC-7721 cells (1.68 nmol/L) was lower than that for L02 cells (5.68 nmol/L), showing a preferential cytotoxicity of rHDL-ACM for SMMC-7721 cells.
Conclusion: rHDL-ACM complex keeps the basic physical and biological binding properties of native HDL and shows a preferential cytotoxicity for SMMC-7721 hepatoma to normal L02 hepatocytes. HDL is a potential carrier for delivering lipophilic antitumoral drug to hepatoma cells.
Figures


Similar articles
-
Delivery of hydrophilic drug doxorubicin hydrochloride-targeted liver using apoAI as carrier.J Drug Target. 2013 May;21(4):367-74. doi: 10.3109/1061186X.2012.757769. Epub 2013 Apr 19. J Drug Target. 2013. PMID: 23600747
-
Recombinant lipoproteins reinforce cytotoxicity of doxorubicin to hepatocellular carcinoma.J Drug Target. 2014 Jan;22(1):76-85. doi: 10.3109/1061186X.2013.839687. Epub 2013 Oct 4. J Drug Target. 2014. PMID: 24093636
-
Targeted delivery of garcinia glycosides by reconstituted high-density lipoprotein nano-complexes.J Microencapsul. 2018 Mar;35(2):115-120. doi: 10.1080/02652048.2017.1413146. Epub 2018 Feb 7. J Microencapsul. 2018. PMID: 29195484
-
Evaluation of reconstituted high-density lipoprotein (rHDL) as a drug delivery platform - a detailed survey of rHDL particles ranging from biophysical properties to clinical implications.Nanomedicine. 2016 Oct;12(7):2161-2179. doi: 10.1016/j.nano.2016.05.009. Epub 2016 May 26. Nanomedicine. 2016. PMID: 27237620 Review.
-
Reconstituted high density lipoprotein-based nanoparticles: an overview of applications in regenerative medicine, preparation, evaluation and future trends.Curr Pharm Des. 2015;21(12):1529-44. doi: 10.2174/1381612821666150115130102. Curr Pharm Des. 2015. PMID: 25594411 Review.
Cited by
-
SR-B1: A Unique Multifunctional Receptor for Cholesterol Influx and Efflux.Annu Rev Physiol. 2018 Feb 10;80:95-116. doi: 10.1146/annurev-physiol-021317-121550. Epub 2017 Nov 10. Annu Rev Physiol. 2018. PMID: 29125794 Free PMC article. Review.
-
Influence of liver cancer on lipid and lipoprotein metabolism.Lipids Health Dis. 2006 Mar 3;5:4. doi: 10.1186/1476-511X-5-4. Lipids Health Dis. 2006. PMID: 16515689 Free PMC article. Review.
-
Role of lipids in spheroidal high density lipoproteins.PLoS Comput Biol. 2010 Oct 28;6(10):e1000964. doi: 10.1371/journal.pcbi.1000964. PLoS Comput Biol. 2010. PMID: 21060857 Free PMC article.
-
Detecting the functional complexities between high-density lipoprotein mimetics.Biomaterials. 2018 Jul;170:58-69. doi: 10.1016/j.biomaterials.2018.04.011. Epub 2018 Apr 6. Biomaterials. 2018. PMID: 29653287 Free PMC article.
-
Lipids changes in liver cancer.J Zhejiang Univ Sci B. 2007 Jun;8(6):398-409. doi: 10.1631/jzus.2007.B0398. J Zhejiang Univ Sci B. 2007. PMID: 17565510 Free PMC article. Review.
References
-
- Firestone RA. Low-density lipoprotein as a vehicle for targeting antitumor compounds to cancer cells. Bioconjug Chem. 1994;5:105–113. - PubMed
-
- de Smidt PC, van Berkel TJ. LDL-mediated drug targeting. Crit Rev Ther Drug Carrier Syst. 1990;7:99–120. - PubMed
-
- Janknegt R. Liposomal formulations of cytotoxic drugs. Support Care Cancer. 1996;4:298–304. - PubMed
-
- Khazaeli MB, Conry RM, LoBuglio AF. Human immune response to monoclonal antibodies. J Immunother Emphasis Tumor Immunol. 1994;15:42–52. - PubMed
-
- Tokui T, Kuroiwa C, Muramatsu S, Tokui Y, Sasagawa K, Ikeda T, Komai T. Plasma lipoproteins as targeting carriers to tumour tissues after administration of a lipophilic agent to mice. Biopharm Drug Dispos. 1995;16:91–103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

