Selective inhibition of HIV-1 reverse transcriptase-associated ribonuclease H activity by hydroxylated tropolones
- PMID: 15741178
- PMCID: PMC552956
- DOI: 10.1093/nar/gki268
Selective inhibition of HIV-1 reverse transcriptase-associated ribonuclease H activity by hydroxylated tropolones
Abstract
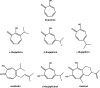
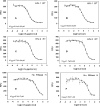
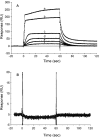
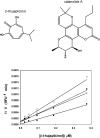
High-throughput screening of a National Cancer Institute library of pure natural products identified the hydroxylated tropolone derivatives beta-thujaplicinol (2,7-dihydroxy-4-1(methylethyl)-2,4,6-cycloheptatrien-1-one) and manicol (1,2,3,4-tetrahydro-5-7-dihydroxy-9-methyl-2-(1-methylethenyl)-6H-benzocyclohepten-6-one) as potent and selective inhibitors of the ribonuclease H (RNase H) activity of human immunodeficiency virus-type 1 reverse transcriptase (HIV-1 RT). beta-Thujaplicinol inhibited HIV-1 RNase H in vitro with an IC50 of 0.2 microM, while the IC50 for Escherichia coli and human RNases H was 50 microM and 5.7 microM, respectively. In contrast, the related tropolone analog beta-thujaplicin (2-hydroxy-4-(methylethyl)-2,4,6-cycloheptatrien-1-one), which lacks the 7-OH group of the heptatriene ring, was inactive, while manicol, which possesses a 7-OH group, inhibited HIV-1 and E.coli RNases H with IC50 = 1.5 microM and 40 microM, respectively. Such a result highlights the importance of the 2,7-dihydroxy function of these tropolone analogs, possibly through a role in metal chelation at the RNase H active site. Inhibition of HIV-2 RT-associated RNase H indirectly indicates that these compounds do not occupy the nonnucleoside inhibitor-binding pocket in the vicinity of the DNA polymerase domain. Both beta-thujaplicinol and manicol failed to inhibit DNA-dependent DNA polymerase activity of HIV-1 RT at a concentration of 50 microM, suggesting that they are specific for the C-terminal RNase H domain, while surface plasmon resonance studies indicated that the inhibition was not due to intercalation of the analog into the nucleic acid substrate. Finally, we have demonstrated synergy between beta-thujaplicinol and calanolide A, a nonnucleoside inhibitor of HIV-1 RT, raising the possibility that both enzymatic activities of HIV-1 RT can be simultaneously targeted.
Figures
Similar articles
-
Inhibition of the ribonuclease H and DNA polymerase activities of HIV-1 reverse transcriptase by N-(4-tert-butylbenzoyl)-2-hydroxy-1-naphthaldehyde hydrazone.Biochemistry. 1997 Mar 18;36(11):3179-85. doi: 10.1021/bi9624696. Biochemistry. 1997. PMID: 9115994
-
Transient kinetic analyses of the ribonuclease H cleavage activity of HIV-1 reverse transcriptase in complex with efavirenz and/or a β-thujaplicinol analogue.Biochem J. 2013 Oct 15;455(2):179-84. doi: 10.1042/BJ20130850. Biochem J. 2013. PMID: 23927736
-
6-[1-(4-Fluorophenyl)methyl-1H-pyrrol-2-yl)]-2,4-dioxo-5-hexenoic acid ethyl ester a novel diketo acid derivative which selectively inhibits the HIV-1 viral replication in cell culture and the ribonuclease H activity in vitro.Antiviral Res. 2005 Feb;65(2):117-24. doi: 10.1016/j.antiviral.2004.11.002. Antiviral Res. 2005. PMID: 15708638
-
The involvement of HIV-1 RNAse H in resistance to nucleoside analogues.J Antimicrob Chemother. 2008 May;61(5):973-5. doi: 10.1093/jac/dkn060. Epub 2008 Mar 5. J Antimicrob Chemother. 2008. PMID: 18325896 Review.
-
Uncovering the complexities of retroviral ribonuclease H reveals its potential as a therapeutic target.AIDS Rev. 2002 Oct-Dec;4(4):183-94. AIDS Rev. 2002. PMID: 12555693 Review.
Cited by
-
Inhibition of the DNA polymerase and RNase H activities of HIV-1 reverse transcriptase and HIV-1 replication by Brasenia schreberi (Junsai) and Petasites japonicus (Fuki) components.J Nat Med. 2015 Jul;69(3):432-40. doi: 10.1007/s11418-015-0885-9. Epub 2015 Feb 8. J Nat Med. 2015. PMID: 25663480
-
Free Energy-Based Virtual Screening and Optimization of RNase H Inhibitors of HIV-1 Reverse Transcriptase.ACS Omega. 2016 Sep 30;1(3):435-447. doi: 10.1021/acsomega.6b00123. Epub 2016 Sep 21. ACS Omega. 2016. PMID: 27713931 Free PMC article.
-
Identification of highly conserved residues involved in inhibition of HIV-1 RNase H function by Diketo acid derivatives.Antimicrob Agents Chemother. 2014 Oct;58(10):6101-10. doi: 10.1128/AAC.03605-14. Epub 2014 Aug 4. Antimicrob Agents Chemother. 2014. PMID: 25092689 Free PMC article.
-
Crystal structures of the reverse transcriptase-associated ribonuclease H domain of xenotropic murine leukemia-virus related virus.J Struct Biol. 2012 Mar;177(3):638-45. doi: 10.1016/j.jsb.2012.02.006. Epub 2012 Feb 16. J Struct Biol. 2012. PMID: 22366278 Free PMC article.
-
Design, synthesis and biological evaluation of 2-quinolyl-1,3-tropolone derivatives as new anti-cancer agents.RSC Adv. 2021;11(8):4555-4571. doi: 10.1039/d0ra10610k. Epub 2021 Jan 22. RSC Adv. 2021. PMID: 33996031 Free PMC article.
References
-
- Klarmann G.J., Hawkins M.E., Le Grice S.F. Uncovering the complexities of retroviral ribonuclease H reveals its potential as a therapeutic target. AIDS Rev. 2002;4:183–194. - PubMed
-
- Schatz O., Cromme F., Naas T., Lindemann D., Gruninger-Leitch F., Mous J., Le Grice S.F.J. Inactivation of the RNaseH domain of HIV-1 reverse transcriptase blocks viral infectivity. In: Papas T., editor. Oncogenesis and AIDS. Houston, TX: Portfolio Publishing Company; 1990. pp. 55–68.
-
- Tisdale M., Schulze T., Larder B.A., Moelling K. Mutations within the RNase H domain of human immunodeficiency virus type 1 reverse transcriptase abolish virus infectivity. J. Gen. Virol. 1991;72:59–66. - PubMed
-
- Parniak M.A., Sluis-Cremer N. Inhibitors of HIV-1 reverse transcriptase. Adv. Pharmacol. 2000;49:67–109. - PubMed
-
- Borkow G., Fletcher R.S., Barnard J., Arion D., Motakis D., Dmitrienko G.I., Parniak M.A. Inhibition of the ribonuclease H and DNA polymerase activities of HIV-1 reverse transcriptase by N-(4-tert-butylbenzoyl)-2-hydroxy-1-naphthaldehyde hydrazone. Biochemistry. 1997;36:3179–3185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

