Latency, chromatin remodeling, and reactivation of human cytomegalovirus in the dendritic cells of healthy carriers
- PMID: 15738399
- PMCID: PMC554799
- DOI: 10.1073/pnas.0408994102
Latency, chromatin remodeling, and reactivation of human cytomegalovirus in the dendritic cells of healthy carriers
Abstract
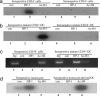
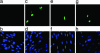
Human cytomegalovirus (HCMV) persists as a subclinical, lifelong infection in the normal human host, but reactivation from latency in immunocompromised subjects results in serious disease. Latency and reactivation are defining characteristics of the herpesviruses and are key to understanding their biology; however, the precise cellular sites in which HCMV is carried and the mechanisms regulating its latency and reactivation during natural infection remain poorly understood. Here we present evidence, based entirely on direct analysis of material isolated from healthy virus carriers, to show that myeloid dendritic cell (DC) progenitors are sites of HCMV latency and that their ex vivo differentiation to a mature DC phenotype is linked with reactivation of infectious virus resulting from differentiation-dependent chromatin remodeling of the viral major immediate-early promoter. Thus, myeloid DC progenitors are a site of HCMV latency during natural persistence, and there is a critical linkage between their differentiation to DC and transcriptional reactivation of latent virus, which is likely to play an important role in the pathogenesis of HCMV infection.
Figures



Similar articles
-
Human cytomegalovirus: Latency and reactivation in the myeloid lineage.J Clin Virol. 2008 Mar;41(3):180-5. doi: 10.1016/j.jcv.2007.11.014. J Clin Virol. 2008. PMID: 18164651 Review.
-
Latency and reactivation of human cytomegalovirus.J Gen Virol. 2006 Jul;87(Pt 7):1763-1779. doi: 10.1099/vir.0.81891-0. J Gen Virol. 2006. PMID: 16760381 Review.
-
An in vitro model for the regulation of human cytomegalovirus latency and reactivation in dendritic cells by chromatin remodelling.J Gen Virol. 2005 Nov;86(Pt 11):2949-2954. doi: 10.1099/vir.0.81161-0. J Gen Virol. 2005. PMID: 16227215
-
Circulating dendritic cells isolated from healthy seropositive donors are sites of human cytomegalovirus reactivation in vivo.J Virol. 2013 Oct;87(19):10660-7. doi: 10.1128/JVI.01539-13. Epub 2013 Jul 24. J Virol. 2013. PMID: 23885077 Free PMC article.
-
Inhibition of inflammatory interleukin-6 activity via extracellular signal-regulated kinase-mitogen-activated protein kinase signaling antagonizes human cytomegalovirus reactivation from dendritic cells.J Virol. 2011 Dec;85(23):12750-8. doi: 10.1128/JVI.05878-11. Epub 2011 Sep 21. J Virol. 2011. PMID: 21937636 Free PMC article.
Cited by
-
Virus-Specific Nanobody-Chimeras Degrade the Human Cytomegalovirus US28 Protein in CD34+ Cells.Pathogens. 2024 Sep 24;13(10):821. doi: 10.3390/pathogens13100821. Pathogens. 2024. PMID: 39452693 Free PMC article.
-
Navigating Latency-Inducing Viral Infections: Therapeutic Targeting and Nanoparticle Utilization.Biomater Res. 2024 Oct 16;28:0078. doi: 10.34133/bmr.0078. eCollection 2024. Biomater Res. 2024. PMID: 39416703 Free PMC article. Review.
-
Breast milk induces the differentiation of monocytes into macrophages, promoting human cytomegalovirus infection.J Virol. 2024 Sep 17;98(9):e0117724. doi: 10.1128/jvi.01177-24. Epub 2024 Aug 28. J Virol. 2024. PMID: 39194236
-
Cytokine Dynamics and Herpesvirus Interactions in Pediatric Liver and Kidney Transplant Recipients: The Distinct Behavior of HCMV, HHV6, HHV7 and EBV.Viruses. 2024 Jul 2;16(7):1067. doi: 10.3390/v16071067. Viruses. 2024. PMID: 39066229 Free PMC article.
-
The Triterpenoid MOMORDIN-Ic Inhibits HCMV by Preventing the Initiation of Gene Expression in Eukaryotic Cells.Pathogens. 2024 Jun 28;13(7):546. doi: 10.3390/pathogens13070546. Pathogens. 2024. PMID: 39057773 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

