Distinct roles of pattern recognition receptors CD14 and Toll-like receptor 4 in acute lung injury
- PMID: 15731076
- PMCID: PMC1064978
- DOI: 10.1128/IAI.73.3.1754-1763.2005
Distinct roles of pattern recognition receptors CD14 and Toll-like receptor 4 in acute lung injury
Erratum in
-
Correction for Jeyaseelan et al., "Distinct Roles of Pattern Recognition Receptors CD14 and Toll-Like Receptor 4 in Acute Lung Injury".Infect Immun. 2022 Apr 21;90(4):e0001422. doi: 10.1128/iai.00014-22. Epub 2022 Mar 14. Infect Immun. 2022. PMID: 35285245 Free PMC article. No abstract available.
Abstract
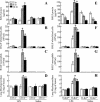
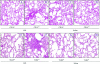
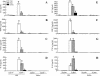
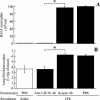
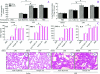
Acute lung injury (ALI) induced by lipopolysaccharide (LPS) is a major cause of mortality among humans. ALI is characterized by microvascular protein leakage, neutrophil influx, and expression of proinflammatory mediators, followed by severe lung damage. LPS binding to its receptors is the crucial step in the causation of these multistep events. LPS binding and signaling involves CD14 and Toll-like receptor 4 (TLR4). However, the relative contributions of CD14 and TLR4 in the induction of ALI and their therapeutic potentials are not clear in vivo. Therefore, the aim of the present study was to compare the roles of CD14 and TLR4 in LPS-induced ALI to determine which of these molecules is the more critical target for attenuating ALI in a mouse model. Our results show that CD14 and TLR4 are necessary for low-dose (300-microg/ml) LPS-induced microvascular leakage, NF-kappaB activation, neutrophil influx, cytokine and chemokine (KC, macrophage inflammatory protein 2, tumor necrosis factor alpha, interleukin-6) expression, and subsequent lung damage. On the other hand, when a 10-fold-higher dose of LPS (3 mg/ml) was used, these responses were only partially dependent on CD14 and they were totally dependent on TLR4. The CD14-independent LPS response was dependent on CD11b. A TLR4 blocking antibody abolished microvascular leakage, neutrophil accumulation, cytokine responses, and lung pathology with a low dose of LPS but only attenuated the responses with a high dose of LPS. These data are the first to demonstrate that LPS-induced CD14-dependent and -independent (CD11b-dependent) signaling pathways in the lung are entirely dependent on TLR4 and that blocking TLR4 might be beneficial in lung diseases caused by LPS from gram-negative pathogens.
Figures
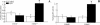


Similar articles
-
Type 1 fimbriae deliver an LPS- and TLR4-dependent activation signal to CD14-negative cells.Mol Microbiol. 2001 Feb;39(3):542-52. doi: 10.1046/j.1365-2958.2001.02205.x. Mol Microbiol. 2001. PMID: 11169097
-
Lipopolysaccharide-induced leukocyte-endothelial cell interactions: a role for CD14 versus toll-like receptor 4 within microvessels.J Immunol. 2002 Aug 15;169(4):2111-9. doi: 10.4049/jimmunol.169.4.2111. J Immunol. 2002. PMID: 12165539
-
Induction of TNF-alpha and MnSOD by endotoxin: role of membrane CD14 and Toll-like receptor-4.Am J Physiol Cell Physiol. 2001 Jun;280(6):C1422-30. doi: 10.1152/ajpcell.2001.280.6.C1422. Am J Physiol Cell Physiol. 2001. PMID: 11350737
-
Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-CD14 and Toll-like receptors.Crit Rev Oral Biol Med. 2002;13(2):132-42. doi: 10.1177/154411130201300204. Crit Rev Oral Biol Med. 2002. PMID: 12097356 Review.
-
[CD14 protein as a modulator of the inflammatory response].Postepy Biochem. 2024 Jan 30;69(4):274-282. doi: 10.18388/pb.2021_501. Print 2024 Jan 30. Postepy Biochem. 2024. PMID: 39012698 Review. Polish.
Cited by
-
Nimbolide protects against endotoxin-induced acute respiratory distress syndrome by inhibiting TNF-α mediated NF-κB and HDAC-3 nuclear translocation.Cell Death Dis. 2019 Jan 28;10(2):81. doi: 10.1038/s41419-018-1247-9. Cell Death Dis. 2019. PMID: 30692512 Free PMC article.
-
Bupleurum polysaccharides attenuates lipopolysaccharide-induced inflammation via modulating Toll-like receptor 4 signaling.PLoS One. 2013 Oct 22;8(10):e78051. doi: 10.1371/journal.pone.0078051. eCollection 2013. PLoS One. 2013. PMID: 24167596 Free PMC article.
-
Association of Toll-like receptor 4 alleles with symptoms and sensitization to laboratory animals.J Allergy Clin Immunol. 2008 Nov;122(5):896-902.e4. doi: 10.1016/j.jaci.2008.08.025. Epub 2008 Oct 4. J Allergy Clin Immunol. 2008. PMID: 18835634 Free PMC article.
-
The anti-inflammatory and antiviral properties of anionic pulmonary surfactant phospholipids.Immunol Rev. 2023 Aug;317(1):166-186. doi: 10.1111/imr.13207. Epub 2023 May 5. Immunol Rev. 2023. PMID: 37144896 Free PMC article. Review.
-
The acute respiratory distress syndrome: from mechanism to translation.J Immunol. 2015 Feb 1;194(3):855-60. doi: 10.4049/jimmunol.1402513. J Immunol. 2015. PMID: 25596299 Free PMC article. Review.
References
-
- Akashi, S., S. Saitoh, Y. Wakabayashi, T. Kikuchi, N. Takamura, Y. Nagai, Y. Kusumoto, K. Fukase, S. Kusumoto, Y. Adachi, A. Kosugi, and K. Miyake. 2003. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14. J. Exp. Med. 198:1035-1042. - PMC - PubMed
-
- Akashi, S., R. Shimazu, H. Ogata, Y. Nagai, K. Takeda, M. Kimoto, and K. Miyake. 2000. Cutting edge: cell surface expression and lipopolysaccharide signaling via the Toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J. Immunol. 164:3471-3475. - PubMed
-
- Andonegui, G., S. M. Goyert, and P. Kubes. 2002. Lipopolysaccharide-induced leukocyte-endothelial cell interactions: a role for CD14 versus Toll-like receptor 4 within microvessels. J. Immunol. 169:2111-2119. - PubMed
-
- Barsness, K. A., J. Arcaroli, A. H. Harken, E. Abraham, A. Banerjee, L. Reznikov, and R. C. McIntyre, Jr. 2004. Hemorrhage-induced acute lung injury is TLR-4 dependent. Am. J. Physiol. Regul. Integr. Comp. Physiol. 87:R592-R599. - PubMed
-
- Bernard, G. R., A. Artigas, K. L. Brigham, J. Carlet, K. Falke, L. Hudson, M. Lamy, J. R. Legall, A. Morris, and R. Spragg. 1994. The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 149:818-824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

