Local phosphatidylinositol 3,4,5-trisphosphate accumulation recruits Vav2 and Vav3 to activate Rac1/Cdc42 and initiate neurite outgrowth in nerve growth factor-stimulated PC12 cells
- PMID: 15728722
- PMCID: PMC1087229
- DOI: 10.1091/mbc.e04-10-0904
Local phosphatidylinositol 3,4,5-trisphosphate accumulation recruits Vav2 and Vav3 to activate Rac1/Cdc42 and initiate neurite outgrowth in nerve growth factor-stimulated PC12 cells
Abstract
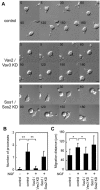
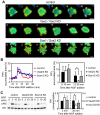
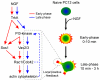
Neurite outgrowth is an important process in the formation of neuronal networks. Rac1 and Cdc42, members of the Rho-family GTPases, positively regulate neurite extension through reorganization of the actin cytoskeleton. Here, we examine the dynamic linkage between Rac1/Cdc42 and phosphatidylinositol 3-kinase (PI3-kinase) during nerve growth factor (NGF)-induced neurite outgrowth in PC12 cells. Activity imaging using fluorescence resonance energy transfer probes showed that PI3-kinase as well as Rac1/Cdc42 was transiently activated in broad areas of the cell periphery immediately after NGF addition. Subsequently, local and repetitive activation of PI3-kinase and Rac1/Cdc42 was observed at the protruding sites. Depletion of Vav2 and Vav3 by RNA interference significantly inhibited both Rac1/Cdc42 activation and the formation of short processes leading to neurite outgrowth. At the NGF-induced protrusions, local phosphatidylinositol 3,4,5-trisphosphate accumulation recruited Vav2 and Vav3 to activate Rac1 and Cdc42, and conversely, Vav2 and Vav3 were required for the local activation of PI3-kinase. These observations demonstrated for the first time that Vav2 and Vav3 are essential constituents of the positive feedback loop that is comprised of PI3-kinase and Rac1/Cdc42 and cycles locally with morphological changes.
Figures
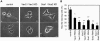

Similar articles
-
Vav Proteins in Development of the Brain: A Potential Relationship to the Pathogenesis of Congenital Zika Syndrome?Viruses. 2022 Feb 14;14(2):386. doi: 10.3390/v14020386. Viruses. 2022. PMID: 35215978 Free PMC article. Review.
-
Phosphorylation of STEF/Tiam2 by protein kinase A is critical for Rac1 activation and neurite outgrowth in dibutyryl cAMP-treated PC12D cells.Mol Biol Cell. 2011 May 15;22(10):1780-90. doi: 10.1091/mbc.E10-09-0783. Epub 2011 Apr 1. Mol Biol Cell. 2011. PMID: 21460187 Free PMC article.
-
Spatio-temporal regulation of Rac1 and Cdc42 activity during nerve growth factor-induced neurite outgrowth in PC12 cells.J Biol Chem. 2004 Jan 2;279(1):713-9. doi: 10.1074/jbc.M306382200. Epub 2003 Oct 21. J Biol Chem. 2004. PMID: 14570905
-
The c-Fes tyrosine kinase cooperates with the breakpoint cluster region protein (Bcr) to induce neurite extension in a Rac- and Cdc42-dependent manner.Exp Cell Res. 2004 Sep 10;299(1):188-98. doi: 10.1016/j.yexcr.2004.05.010. Exp Cell Res. 2004. PMID: 15302586
-
The role of Rho GTPases and associated kinases in regulating neurite outgrowth.Int J Biochem Cell Biol. 2002 Jul;34(7):731-45. doi: 10.1016/s1357-2725(01)00167-4. Int J Biochem Cell Biol. 2002. PMID: 11950591 Review.
Cited by
-
Vav Proteins in Development of the Brain: A Potential Relationship to the Pathogenesis of Congenital Zika Syndrome?Viruses. 2022 Feb 14;14(2):386. doi: 10.3390/v14020386. Viruses. 2022. PMID: 35215978 Free PMC article. Review.
-
Role of Cdc42 in neurite outgrowth of PC12 cells and cerebellar granule neurons.Mol Cell Biochem. 2006 Jan;281(1-2):17-25. doi: 10.1007/s11010-006-0165-9. Mol Cell Biochem. 2006. PMID: 16328953
-
Expression of USP25 associates with fibrosis, inflammation and metabolism changes in IgG4-related disease.Nat Commun. 2024 Mar 23;15(1):2627. doi: 10.1038/s41467-024-45977-7. Nat Commun. 2024. PMID: 38521787 Free PMC article.
-
New potent accelerator of neurite outgrowth from Lawsonia inermis flower under non-fasting condition.J Nat Med. 2016 Jul;70(3):384-90. doi: 10.1007/s11418-016-0974-4. Epub 2016 Mar 2. J Nat Med. 2016. PMID: 26936787
-
Telencephalin protects PAJU cells from amyloid beta protein-induced apoptosis by activating the ezrin/radixin/moesin protein family/phosphatidylinositol-3-kinase/protein kinase B pathway.Neural Regen Res. 2012 Oct 5;7(28):2189-98. doi: 10.3969/j.issn.1673-5374.2012.028.004. Neural Regen Res. 2012. PMID: 25538739 Free PMC article.
References
-
- Angelastro, J. M., Klimaschewski, L., Tang, S., Vitolo, O. V., Weissman, T. A., Donlin, L. T., Shelanski, M. L., and Greene, L. A. (2000). Identification of diverse nerve growth factor-regulated genes by serial analysis of gene expression (SAGE) profiling. Proc. Natl. Acad. Sci. USA 97, 10424–10429. - PMC - PubMed
-
- Aoki, K., Nakamura, T., and Matsuda, M. (2004). Spatio-temporal regulation of Rac1 and Cdc42 activity during nerve growth factor-induced neurite outgrowth in PC12 cells. J. Biol. Chem. 279, 713–719. - PubMed
-
- Bibel, M., and Barde, Y. A. (2000). Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 14, 2919–2937. - PubMed
-
- Bourne, H. R., and Weiner, O. (2002). A chemical compass. Nature 419, 21. - PubMed
-
- Brummelkamp, T. R., Bernards, R., and Agami, R. (2002). A system for stable expression of short interfering RNAs in mammalian cells. Science 296, 550–553. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

