Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation
- PMID: 15716400
- PMCID: PMC6725934
- DOI: 10.1523/JNEUROSCI.3269-04.2005
Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation
Abstract
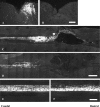
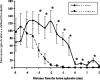
Sensory axons in the adult spinal cord do not regenerate after injury. This is essentially because of inhibitory components in the damaged CNS, such as myelin-associated inhibitors and the glial scar. However, if the sciatic nerve is axotomized before injury of the dorsal column, injured axons can regenerate a short distance in the spinal cord. Here, we show that sciatic nerve transection results in time-dependent phosphorylation and activation of the transcription factor, signal transducer and activator of transcription 3 (STAT3), in dorsal root ganglion (DRG) neurons. This effect is specific to peripheral injuries and does not occur when the dorsal column is crushed. Sustained perineural infusion of the Janus kinase 2 (JAK2) inhibitor AG490 to the proximal nerve stump can block STAT3 phosphorylation after sciatic nerve transection and results in reduced growth-associated protein 43 upregulation and compromised neurite outgrowth in vitro. Importantly, in vivo perineural infusion of AG490 also significantly attenuates dorsal column axonal regeneration in the adult spinal cord after a preconditioning sciatic nerve transection. We conclude that STAT3 activation is necessary for increased growth ability of DRG neurons and improved axonal regeneration in the spinal cord after a conditioning injury.
Figures
Similar articles
-
Conditioning injury-induced spinal axon regeneration fails in interleukin-6 knock-out mice.J Neurosci. 2004 May 5;24(18):4432-43. doi: 10.1523/JNEUROSCI.2245-02.2004. J Neurosci. 2004. PMID: 15128857 Free PMC article.
-
Peripheral but not central axotomy induces changes in Janus kinases (JAK) and signal transducers and activators of transcription (STAT).Eur J Neurosci. 2000 Apr;12(4):1165-76. doi: 10.1046/j.1460-9568.2000.00005.x. Eur J Neurosci. 2000. PMID: 10762348
-
Injury-induced Janus kinase/protein kinase C-dependent phosphorylation of growth-associated protein 43 and signal transducer and activator of transcription 3 for neurite growth in dorsal root ganglion.J Neurosci Res. 2007 Feb 1;85(2):321-31. doi: 10.1002/jnr.21119. J Neurosci Res. 2007. PMID: 17131417
-
The Dorsal Column Lesion Model of Spinal Cord Injury and Its Use in Deciphering the Neuron-Intrinsic Injury Response.Dev Neurobiol. 2018 Oct;78(10):926-951. doi: 10.1002/dneu.22601. Epub 2018 May 11. Dev Neurobiol. 2018. PMID: 29717546 Free PMC article. Review.
-
Overcoming inhibition in the damaged spinal cord.J Neurotrauma. 2006 Mar-Apr;23(3-4):371-83. doi: 10.1089/neu.2006.23.371. J Neurotrauma. 2006. PMID: 16629623 Review.
Cited by
-
The nuclear events guiding successful nerve regeneration.Front Mol Neurosci. 2011 Dec 12;4:53. doi: 10.3389/fnmol.2011.00053. eCollection 2011. Front Mol Neurosci. 2011. PMID: 22180737 Free PMC article.
-
Leptin inhibits 4-aminopyridine- and pentylenetetrazole-induced seizures and AMPAR-mediated synaptic transmission in rodents.J Clin Invest. 2008 Jan;118(1):272-80. doi: 10.1172/JCI33009. J Clin Invest. 2008. PMID: 18097472 Free PMC article.
-
Signaling to transcription networks in the neuronal retrograde injury response.Sci Signal. 2010 Jul 13;3(130):ra53. doi: 10.1126/scisignal.2000952. Sci Signal. 2010. PMID: 20628157 Free PMC article.
-
Therapeutic potential of hepatocyte growth factor against cerebral ischemia (Review).Exp Ther Med. 2015 Feb;9(2):283-288. doi: 10.3892/etm.2014.2133. Epub 2014 Dec 15. Exp Ther Med. 2015. PMID: 25574187 Free PMC article.
-
An evolutionarily conserved mechanism for cAMP elicited axonal regeneration involves direct activation of the dual leucine zipper kinase DLK.Elife. 2016 Jun 7;5:e14048. doi: 10.7554/eLife.14048. Elife. 2016. PMID: 27268300 Free PMC article.
References
-
- Aaronson DS, Horvath CM (2002) A road map for those who don't know JAK-STAT. Science 296: 1653-1655. - PubMed
-
- Andersen LB, Schreyer DJ (1999) Constitutive expression of GAP-43 correlates with rapid, but not slow regrowth of injured dorsal root axons in the adult rat. Exp Neurol 155: 157-164. - PubMed
-
- Bolin LM, Verity AN, Silver JE, Shooter EM, Abrams JS (1995) Interleukin-6 production by Schwann cells and induction in sciatic nerve injury. J Neurochem 64: 850-858. - PubMed
-
- Bomze HM, Bulsara KR, Iskandar BJ, Caroni P, Skene JH (2001) Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nat Neurosci 4: 38-43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
