Inhibition of endothelial cell migration by thrombospondin-1 type-1 repeats is mediated by beta1 integrins
- PMID: 15716381
- PMCID: PMC2171765
- DOI: 10.1083/jcb.200407060
Inhibition of endothelial cell migration by thrombospondin-1 type-1 repeats is mediated by beta1 integrins
Erratum in
- J Cell Biol. 2005 May 9;169(3):541
Abstract
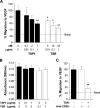
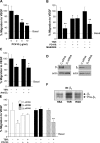
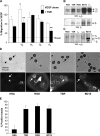
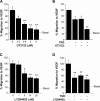
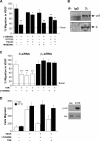
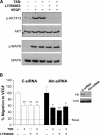
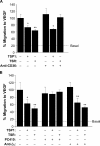
The anti-angiogenic effect of thrombospondin-1 has been shown to be mediated through binding of the type-1 repeat (TSR) domain to the CD36 transmembrane receptor. We now report that the TSR domain can inhibit VEGF-induced migration in human umbilical vein endothelial cells (HUVEC), cells that lack CD36. Moreover, we identified beta1 integrins as a critical receptor in TSR-mediated inhibition of migration in HUVEC. Using pharmacological inhibitors of downstream VEGF receptor effectors, we found that phosphoinositide 3-kinase (PI3k) was essential for TSR-mediated inhibition of HUVEC migration, but that neither PLCgamma nor Akt was necessary for this response. Furthermore, beta1 integrins were critical for TSR-mediated inhibition of microvascular endothelial cells, cells that express CD36. Together, our results indicate that beta1 integrins mediate the anti-migratory effects of TSR through a PI3k-dependent mechanism.
Figures
Similar articles
-
Oxytocin stimulates in vitro angiogenesis via a Pyk-2/Src-dependent mechanism.Exp Cell Res. 2009 Nov 1;315(18):3210-9. doi: 10.1016/j.yexcr.2009.06.022. Epub 2009 Jun 27. Exp Cell Res. 2009. PMID: 19563802
-
Integrin-linked kinase regulates vascular morphogenesis induced by vascular endothelial growth factor.J Cell Sci. 2004 Jan 26;117(Pt 3):407-15. doi: 10.1242/jcs.00871. Epub 2003 Dec 16. J Cell Sci. 2004. PMID: 14679308
-
Thrombospondin-1 modulates VEGF signaling via CD36 by recruiting SHP-1 to VEGFR2 complex in microvascular endothelial cells.Blood. 2013 Sep 5;122(10):1822-32. doi: 10.1182/blood-2013-01-482315. Epub 2013 Jul 29. Blood. 2013. PMID: 23896411 Free PMC article.
-
CD36: a critical anti-angiogenic receptor.Front Biosci. 2003 Sep 1;8:s874-82. doi: 10.2741/1168. Front Biosci. 2003. PMID: 12957861 Review.
-
CD36-TSP-HRGP interactions in the regulation of angiogenesis.Curr Pharm Des. 2007;13(35):3559-67. doi: 10.2174/138161207782794185. Curr Pharm Des. 2007. PMID: 18220792 Review.
Cited by
-
Exploring the impact of naltrexone on the THBS1/eNOS/NO pathway in osteoporotic bile duct-ligated rats.Sci Rep. 2024 Jan 2;14(1):48. doi: 10.1038/s41598-023-50547-w. Sci Rep. 2024. PMID: 38167957 Free PMC article.
-
ECFC-derived exosomal THBS1 mediates angiogenesis and osteogenesis in distraction osteogenesis via the PI3K/AKT/ERK pathway.J Orthop Translat. 2022 Sep 23;37:12-22. doi: 10.1016/j.jot.2022.08.004. eCollection 2022 Nov. J Orthop Translat. 2022. PMID: 36196150 Free PMC article.
-
Thrombospondins use the VLDL receptor and a nonapoptotic pathway to inhibit cell division in microvascular endothelial cells.Mol Biol Cell. 2008 Feb;19(2):563-71. doi: 10.1091/mbc.e07-07-0649. Epub 2007 Nov 21. Mol Biol Cell. 2008. PMID: 18032585 Free PMC article.
-
Predictive Approach Identifies Molecular Targets and Interventions to Restore Angiogenesis in Wounds With Delayed Healing.Front Physiol. 2019 May 28;10:636. doi: 10.3389/fphys.2019.00636. eCollection 2019. Front Physiol. 2019. PMID: 31191342 Free PMC article.
-
Cyclic stretch affects pulmonary endothelial cell control of pulmonary smooth muscle cell growth.Am J Respir Cell Mol Biol. 2008 Jul;39(1):105-12. doi: 10.1165/rcmb.2007-0283OC. Epub 2008 Feb 28. Am J Respir Cell Mol Biol. 2008. PMID: 18314539 Free PMC article.
References
-
- Asch, A.S., S. Silbiger, E. Heimer, and R.L. Nachman. 1992. Thrombospondin sequence motif (CSVTCG) is responsible for CD36 binding. Biochem. Biophys. Res. Commun. 182:1208–1217. - PubMed
-
- Bjorndahl, M., R. Cao, A. Eriksson, and Y. Cao. 2004. Blockage of VEGF-induced angiogenesis by preventing VEGF secretion. Circ. Res. 94:1443–1450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

