The splenic microenvironment is a source of proangiogenesis/inflammatory mediators accelerating the expansion of murine erythroleukemic cells
- PMID: 15701719
- PMCID: PMC1895028
- DOI: 10.1182/blood-2004-08-3210
The splenic microenvironment is a source of proangiogenesis/inflammatory mediators accelerating the expansion of murine erythroleukemic cells
Abstract
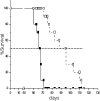
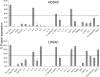
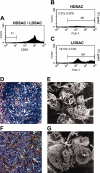
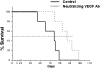
The stromal compartments of hematopoietic organs (eg, spleen) are known to influence the viability and growth of diseased hematopoietic progenitors. Here we have used Friend murine leukemia virus (F-MuLV)-induced erythroleukemia to investigate factors of the splenic microenvironment that may make it fertile for the expansion and survival of malignant erythroblasts. We found that splenectomized, erythroleukemic mice exhibited extended survival compared with age-matched sham controls. In vitro, the proliferation of primary erythroleukemic cells cocultured with leukemic-derived splenic adherent cells or their conditioned media was found to be significantly higher than that observed in cocultures with healthy-derived adherent splenic cells. Cytokine protein arrays revealed that F-MuLV-infected splenocytes secreted elevated levels of interleukin-6 (IL-6), vascular endothelial growth factor-A (VEGF-A), macrophage chemoattractant protein-5 (MCP-5), soluble tumor necrosis factor receptor-1 (sTNFR1), IL-12p70, tumor necrosis factor-alpha (TNF-alpha), and IL-2 over normal splenocytes. Medium supplemented with both VEGF-A and MCP-5 could sustain proliferation of primary erythroleukemic cells in vitro, and significant proliferative suppression was observed upon addition of neutralizing antibodies to either of these factors. Furthermore, in vivo administration of a neutralizing antibody to VEGF-A extended survival times of erythroleukemic mice in comparison with controls. These findings suggest that VEGF-A and MCP-5 are potentially pivotal paracrine mediators occurring within the diseased splenic microenvironment capable of promoting disease acceleration and expansion of erythroleukemic blasts.
Figures
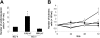

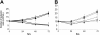
Similar articles
-
Enhanced natural-killer cell and erythropoietic activities in VEGF-A-overexpressing mice delay F-MuLV-induced erythroleukemia.Blood. 2007 Mar 1;109(5):2139-46. doi: 10.1182/blood-2005-11-026823. Epub 2006 Oct 19. Blood. 2007. PMID: 17053052 Free PMC article.
-
Bcl-2 expression in F-MuLV-induced erythroleukemias: a role for the anti-apoptotic action of Bcl-2 during tumor progression.Oncogene. 2001 Apr 26;20(18):2291-300. doi: 10.1038/sj.onc.1204348. Oncogene. 2001. PMID: 11402324
-
p53-independent tumor growth and in vitro cell survival for F-MuLV-induced erythroleukemias.Cell Growth Differ. 1996 Dec;7(12):1651-60. Cell Growth Differ. 1996. PMID: 8959333
-
Erythroleukemia induction by replication-competent type C viruses cloned from the anemia- and polycythemia-inducing isolates of Friend leukemia virus.J Exp Med. 1980 Jun 1;151(6):1493-503. doi: 10.1084/jem.151.6.1493. J Exp Med. 1980. PMID: 6247414 Free PMC article.
-
Targeting tumor micro-environment for design and development of novel anti-angiogenic agents arresting tumor growth.Prog Biophys Mol Biol. 2013 Nov;113(2):333-54. doi: 10.1016/j.pbiomolbio.2013.10.001. Epub 2013 Oct 15. Prog Biophys Mol Biol. 2013. PMID: 24139944 Review.
Cited by
-
Enhanced natural-killer cell and erythropoietic activities in VEGF-A-overexpressing mice delay F-MuLV-induced erythroleukemia.Blood. 2007 Mar 1;109(5):2139-46. doi: 10.1182/blood-2005-11-026823. Epub 2006 Oct 19. Blood. 2007. PMID: 17053052 Free PMC article.
-
Chronic psychological stress activates BMP4-dependent extramedullary erythropoiesis.J Cell Mol Med. 2014 Jan;18(1):91-103. doi: 10.1111/jcmm.12167. Epub 2013 Nov 27. J Cell Mol Med. 2014. PMID: 24283209 Free PMC article.
-
Overexpression of Fli-1 in astrocytoma is associated with poor prognosis.Oncotarget. 2017 Apr 25;8(17):29174-29186. doi: 10.18632/oncotarget.16303. Oncotarget. 2017. PMID: 28418872 Free PMC article.
-
Optimal biologic dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity.Blood. 2005 Nov 1;106(9):3058-61. doi: 10.1182/blood-2005-04-1422. Epub 2005 Jul 5. Blood. 2005. PMID: 15998832 Free PMC article.
-
Quantitative tumor burden imaging parameters of the spleen at MRI for predicting treatment response in patients with acute leukemia.Heliyon. 2023 Sep 21;9(10):e20348. doi: 10.1016/j.heliyon.2023.e20348. eCollection 2023 Oct. Heliyon. 2023. PMID: 37810872 Free PMC article.
References
-
- Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411: 375-379. - PubMed
-
- Wernert N. The multiple roles of tumour stroma. Virchows Arch. 1997;430: 433-443. - PubMed
-
- Litwin C, Leong KG, Zapf R, Sutherland H, Naiman SC, Karsan A. Role of the microenvironment in promoting angiogenesis in acute myeloid leukemia. Am J Hematol. 2002;70: 22-30. - PubMed
-
- Rafii S, Mohle R, Shapiro F, Frey BM, Moore MA. Regulation of hematopoiesis by microvascular endothelium. Leuk Lymphoma. 1997;27: 375-386. - PubMed
-
- Goldman JM, Nolasco I. The spleen in myeloproliferative disorders. Clin Haematol. 1983;12: 505-516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

