Rapid ubiquitination of Syk following GPVI activation in platelets
- PMID: 15701717
- PMCID: PMC1895068
- DOI: 10.1182/blood-2004-09-3689
Rapid ubiquitination of Syk following GPVI activation in platelets
Abstract
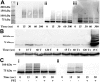
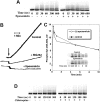
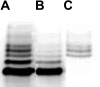
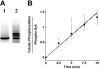
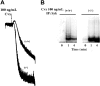
Spleen tyrosine kinase (Syk) activation is a key intermediate step in the activation of platelets by the physiologic agonist collagen. We have found that Syk is rapidly ubiquitinated upon activation of platelets by collagen, collagen-related peptide (CRP), and convulxin. The Src family kinase inhibitors prevented Syk phosphorylation and its ubiquitination, indicating that the process is downstream of Src kinases. The ubiquitination of Syk did not cause degradation of the protein as evidenced by the lack of effect of proteasomal and lysosomal inhibitors. We separated ubiquitinated Syk from its nonubiquitinated counterpart and used an in vitro kinase assay to compare their activities. We found that the ubiquitinated Syk appeared to be about 5-fold more active. Using a phosphospecific antibody to Syk (Tyr525/Tyr526) that measures activated Syk, we found that most (60%-75%) of the active Syk is in the ubiquitinated fraction. This result explains the apparent high specific activity of ubiquitinated Syk. In c-Cbl-deficient mice, Syk is not ubiquitinated, implicating c-Cbl as the E3 ligase involved in Syk ubiquitination. Furthermore, Syk is not dephosphorylated in these mice. We propose that c-Cbl plays a regulatory role in glycoprotein VI (GPVI)/Fc receptor gamma (FcRgamma)-chain-dependent platelet activation through its interaction with Syk.
Figures

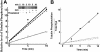
Similar articles
-
Aggretin, a heterodimeric C-type lectin from Calloselasma rhodostoma (Malayan pit viper), stimulates platelets by binding to α2β1 integrin and glycoprotein Ib, activating Syk and phospholipase Cγ 2, but does not involve the glycoprotein VI/Fc receptor γ chain collagen receptor.J Biol Chem. 2001 Jun 15;276(24):20882-9. doi: 10.1074/jbc.M101585200. Epub 2001 Apr 3. J Biol Chem. 2001. PMID: 11287424
-
Collagen-stimulated activation of Syk but not c-Src is severely compromised in human platelets lacking membrane glycoprotein VI.J Biol Chem. 1997 Jan 3;272(1):63-8. doi: 10.1074/jbc.272.1.63. J Biol Chem. 1997. PMID: 8995228
-
Fgr but not Syk tyrosine kinase is a target for beta 2 integrin-induced c-Cbl-mediated ubiquitination in adherent human neutrophils.Biochem J. 2003 Mar 1;370(Pt 2):687-94. doi: 10.1042/BJ20021201. Biochem J. 2003. PMID: 12435267 Free PMC article.
-
Platelet activation mediated through membrane glycoproteins: involvement of tyrosine kinases.Semin Thromb Hemost. 2000;26(1):47-51. doi: 10.1055/s-2000-9803. Semin Thromb Hemost. 2000. PMID: 10805282 Review.
-
GPVI and integrin alphaIIb beta3 signaling in platelets.J Thromb Haemost. 2005 Aug;3(8):1752-62. doi: 10.1111/j.1538-7836.2005.01429.x. J Thromb Haemost. 2005. PMID: 16102042 Review.
Cited by
-
Determination of the substrate specificity of protein-tyrosine phosphatase TULA-2 and identification of Syk as a TULA-2 substrate.J Biol Chem. 2010 Oct 8;285(41):31268-76. doi: 10.1074/jbc.M110.114181. Epub 2010 Jul 29. J Biol Chem. 2010. PMID: 20670933 Free PMC article.
-
Primary platelet signaling cascades and integrin-mediated signaling control ADP-ribosylation factor (Arf) 6-GTP levels during platelet activation and aggregation.J Biol Chem. 2008 May 2;283(18):11995-2003. doi: 10.1074/jbc.M800146200. Epub 2008 Mar 7. J Biol Chem. 2008. PMID: 18326492 Free PMC article.
-
Ubiquitin ligase Cbl-b is a negative regulator for insulin-like growth factor 1 signaling during muscle atrophy caused by unloading.Mol Cell Biol. 2009 Sep;29(17):4798-811. doi: 10.1128/MCB.01347-08. Epub 2009 Jun 22. Mol Cell Biol. 2009. PMID: 19546233 Free PMC article.
-
Global proteome analysis identifies active immunoproteasome subunits in human platelets.Mol Cell Proteomics. 2014 Dec;13(12):3308-19. doi: 10.1074/mcp.M113.031757. Epub 2014 Aug 21. Mol Cell Proteomics. 2014. PMID: 25146974 Free PMC article.
-
Syk and pTyr'd: Signaling through the B cell antigen receptor.Biochim Biophys Acta. 2009 Jul;1793(7):1115-27. doi: 10.1016/j.bbamcr.2009.03.004. Epub 2009 Mar 21. Biochim Biophys Acta. 2009. PMID: 19306898 Free PMC article. Review.
References
-
- Packham MA. Role of platelets in thrombosis and hemostasis. Can J Physiol Pharmacol. 1994;72: 278-284. - PubMed
-
- Santoro SA, Zutter MM. The Alpha2Beta1 integrin: a collagen receptor on platelets and other cells. Thromb Haemost. 1995;74: 813-821. - PubMed
-
- Tsuji M, Ezumi Y, Arai M, Takayama H. A novel association of Fc receptor gamma-chain with glycoprotein VI and their co-expression as a collagen receptor in human platelets. J Biol Chem. 1997;272: 23528-23531. - PubMed
-
- Berlanga O, Bobe R, Becker M, et al. Expression of the collagen receptor glycoprotein VI during megakaryocyte differentiation. Blood. 2000;96: 2740-2745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

